N-Nitroso-Apixaban Impurity B: A Key Reference Standard in Apixaban Impurity Profiling
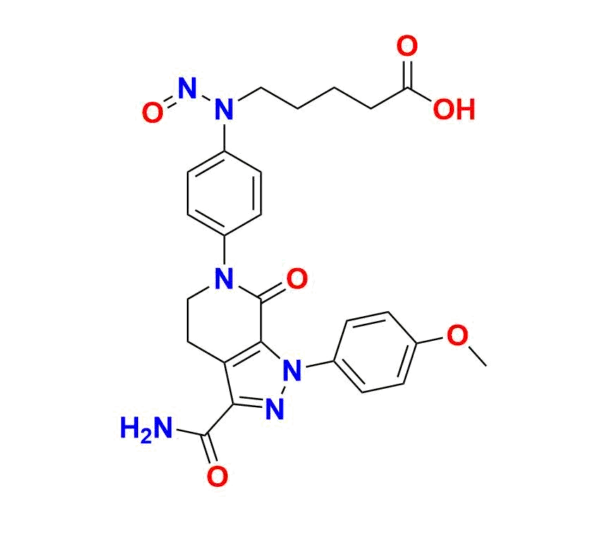
The pharmaceutical industry is witnessing increasing regulatory scrutiny concerning nitrosamine impurities due to their potential carcinogenic risks. One such significant impurity in the synthesis and degradation pathways of Apixaban is N-Nitroso-Apixaban impurity B. It plays a crucial role in analytical research and regulatory compliance for manufacturers of Apixaban-based products.
You can explore this impurity in detail and procure the reference standard from Aquigen Bio Sciences, a leading supplier of high-purity impurity standards.
What is N-Nitroso-Apixaban Impurity B?
N-Nitroso-Apixaban impurity B is a nitrosamine impurity identified in the synthesis pathway of Apixaban, a potent oral anticoagulant. Nitrosamines like this impurity have drawn attention due to their potential genotoxic effects. Regulatory agencies, including the EMA and USFDA, mandate strict control of such impurities, often in the parts-per-million (ppm) range.
Aquigen offers this impurity with:
- ≥98% purity
- Comprehensive documentation (COA, MSDS, NMR, IR, HPLC)
- Availability for both R&D and commercial use
- Custom packaging on request
To learn more or request a quote, visit: N-Nitroso-Apixaban impurity B
Applications and Importance in Pharma
- Impurity profiling in Active Pharmaceutical Ingredient (API) development
- Method development and validation for HPLC/UPLC/MS
- Genotoxic risk assessment and control strategy implementation
- Supporting regulatory submissions such as ANDA, DMF, and CEP
Due to the potential health hazards associated with nitrosamines, strict analytical monitoring is required throughout the manufacturing lifecycle.
Related Apixaban Impurities You Should Know
For a holistic impurity profile of Apixaban, it’s important to consider other process and degradation-related impurities. Aquigen Bio Sciences provides several other critical reference standards that support your analytical and regulatory workflows.
1. Apixaban Amino Acid Impurity
This impurity arises as an intermediate in the synthetic route of Apixaban. It is commonly used during early-stage impurity identification, qualification, and routine batch release testing. The availability of this impurity as a reference standard aids in accurate quantification and validation of impurity levels in final drug substances.
2. Apixaban Carboxylic Acid Impurity
This impurity is another important by-product that can form during hydrolysis or as a degradation product under stressed conditions. It provides insights into the stability profile of Apixaban and is integral to forced degradation studies and stability-indicating method development.
3. Apixaban Amino Acid Impurity Hydrochloride
This hydrochloride salt form of the amino acid impurity is useful for method calibration and improved solubility in specific analytical environments. It supports advanced chromatographic and spectrometric analysis in complex formulations.
Why Choose Aquigen Bio Sciences?
Aquigen Bio Sciences is a trusted name in the field of pharmaceutical impurity standards. Here’s what makes them a preferred partner for global pharma manufacturers:
- High-purity reference standards (≥98%)
- Global shipping with temperature-sensitive logistics
- Complete characterization data for regulatory support
- Customization options for bulk or unique packaging needs
- Quick turnaround times and dedicated technical support
Each impurity is developed under strict quality controls and with regulatory traceability in mind, making them suitable for both early-stage development and post-approval changes.
Final Thoughts
The presence of N-Nitroso-Apixaban impurity B underscores the growing need for advanced impurity detection and quantification strategies in modern pharmaceutical manufacturing. With the tightening of regulatory limits on nitrosamines, access to well-characterized reference standards is not just beneficial — it’s essential.
Aquigen Bio Sciences supports your pharmaceutical quality assurance goals with a wide portfolio of high-purity standards, including:
- Apixaban Amino Acid Impurity
- Apixaban Carboxylic Acid Impurity
- Apixaban Amino Acid Impurity Hydrochloride
To ensure precision and compliance in your Apixaban impurity profiling, visit N-Nitroso-Apixaban impurity B and explore our comprehensive range of standards.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Juegos
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- IT, Cloud, Software and Technology


