Rapid Diagnostics Market Poised to Witness High Growth Due to Increasing Demand for Point-of-Care Testing
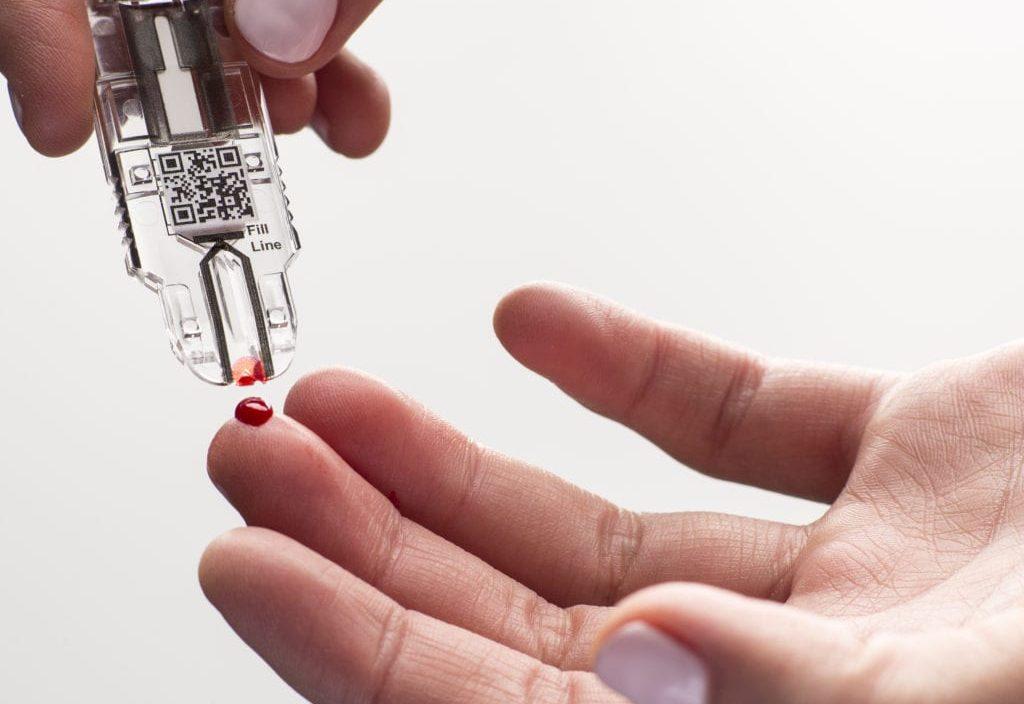
Rapid diagnostics provide quick medical diagnostic tests that are performed near or at the site where the patient is located. These tests are used for conditions such as cardiac markers, infectious diseases, drugs of abuse and hormones testing. Rapid diagnostic tests deliver results within minutes and eliminate the need for sending samples to a laboratory. Common rapid testing devices include lateral flow assays, agglutination assays, immunochromatographic assays and microfluidic-based assays.
The global rapid diagnostics market is estimated to be valued at US$ 80.96 Bn in 2024 and is expected to exhibit a CAGR of 8.2% over the forecast period 2024 to 2031, as highlighted in a new report published by Coherent Market Insights.
The global rapid diagnostics market is estimated to be valued at US$ 80.96 Bn in 2024 and is expected to exhibit a CAGR of 8.2% over the forecast period 2024 to 2031, as highlighted in a new report published by Coherent Market Insights.
Market Dynamics:
Increasing demand for point-of-care testing is estimated to drive the growth of the rapid diagnostics market during the forecast period. Point-of-care tests enable quick detection of diseases and are conducted near the site of patient care. They provide test results within hours instead of sending samples to a centralized laboratory. This not only saves time but also provides convenience to patients. Rapid diagnostic devices have helped address the issues of test result waiting time and delays in diagnostics. Additionally, they help in timely clinical decisions and administering appropriate treatment. However, stringent regulatory approvals and quality assurance are some factors hampering the growth of the market.
SWOT Analysis
Strength: Rapid Diagnostics Market has strong growth potential due to rising demand for POC testing and decentralization of healthcare systems. The increasing awareness about early disease diagnosis is also driving more patients to opt for rapid testing. Further, technological advancements are leading to the development of user-friendly and portable rapid diagnostic kits.
Weakness: High costs associated with manufacturing and R&D of rapid diagnostic devices is a major challenge especially for small players. Unstable regulatory guidelines across regions also act as a roadblock for companies.
Opportunity: Growing geriatric population susceptible to various target conditions present an enormous patient pool. Rising healthcare expenditures in emerging nations will further fuel the adoption of rapid testing. Point-of-care testing delivers fast results and provides convenient access in rural locations.
Threats: Stringent regulations for approval and reliability issues associated with some rapid tests are key threats. Reimbursement policies still favor traditional diagnostic methods in certain regions.
Key Takeaways
Global Rapid Diagnostics Market Size is expected to witness high growth at a CAGR of 8.2% during the forecast period of 2024 to 2031. The market size is projected to increase from US$ 80.96 Bn in 2024 to over US$ 150 Bn by 2031.
Regional analysis: North America currently dominates the market owing to supportive insurance and government policies for diagnostics. The Asia Pacific region is anticipated to showcase rapid growth in the coming years led by India, China, and Japan. Significant unmet needs, rising healthcare investment, and growing medical tourism are driving the regional market.
Key players: Key players operating in the Rapid Diagnostics Market are Nxp Semiconductors N.V., Analog Devices, Inc., and Knowles Corporation. These leading diagnostics companies have wide geographic presence and continuously invest in R&D to develop novel platforms for disease screening and management.
Get More Insights On This Topic: https://www.newswirestats.com/rapid-diagnostics-market-is-expected-to-be-flourished-by-high-demand-for-poc/
Explore More Related Article: https://masstamilan.tv/nasal-sprays-in-the-united-states-an-overview/
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- الألعاب
- Gardening
- Health
- الرئيسية
- Literature
- Music
- Networking
- أخرى
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- IT, Cloud, Software and Technology


