Subacute & Subchronic Toxicity
The Biocompatibility Subacute and Subchronic Toxicity test is used to evaluate the toxicity effects likely to arise from repeated exposures would have on a patient, including any compound toxicity effects. This test determines the systemic effect of repeated doses of materials or their extracts for no less than 24 hours and no greater than 10% of the total lifespan of the test animal.
Subacute toxicity (repeat-dose toxicity) focuses on adverse effects occurring after a single or repeated exposures to a test material per day during a period of 14 to 28 days. Subchronic toxicity indicates the adverse effects of a substance resulting from repeated exposure to a toxic agent over a period of several weeks or months. The subacute and subchronic toxicity studies include full clinical pathology (clinical chemistry, hematology, and coagulation), necropsy and organ weights, as well as histopathology. These tests can either be conducted with full histopathology or limited tissue evaluation.
Standard for Subacute & Subchronic Toxicity Testing
"Tests for systemic toxicity", part eleven of the Biological evaluation of medical devices standards(ISO 10993-11), gives out the general considerations that should be taken into account when evaluating the potential of inducing subacute/ subchronic toxicity of a medical device. It outlines the procedure of studies to assess the potential of medical devices and their constituent materials to induce systemic toxicity.

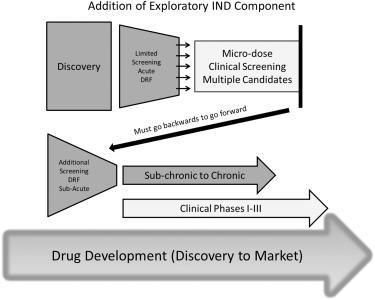
Fig. 1 Subacute & subchronic toxicity tests during drug development. (Denny, 2017)
The usual animals of choice for the subacute and subchronic toxicity tests are mice, rats, or rabbits, and oral, dermal, inhalation, intravenous, intraperitoneal, or subcutaneous application of the test substance may be used based on the considered application of the biomaterial.
General Procedures of Subacute & Subchronic Toxicity Testing
· Administering mice or rats with a dose of 0.9% normal saline or cottonseed extract of the test biomaterial over a 14-day period;
· Observing the test animals once daily for signs of toxicity;
· Recording animal weights on Day 0, Day 7, and Day 14;
· On day 14, collecting blood samples for hematology and clinical chemistry analysis;
· Conducting a gross necropsy and collecting lesions.
STEMart offers biocompatibility Subacute & Subchronic Toxicity testing following the biocompatibility guidelines modified for medical devices. The testing is designed and performed based on the route of exposure to the body including the oral, dermal, and inhalation exposures.
If you have additional questions about Subacute & Subchronic Toxicity testing or would like to find out more about our services, please feel free to contact us.
Reference
Denny, Kevin H., et al. "Acute, subacute, subchronic, and chronic general toxicity testing for preclinical drug development." A Comprehensive Guide to Toxicology in Nonclinical Drug Development 109-127(2017).
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jeux
- Gardening
- Health
- Domicile
- Literature
- Music
- Networking
- Autre
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- IT, Cloud, Software and Technology


