Understanding The Dynamics Of The Global NPHP5 Retinal Degeneration Treatment Market: Market Analysis And Forecasts
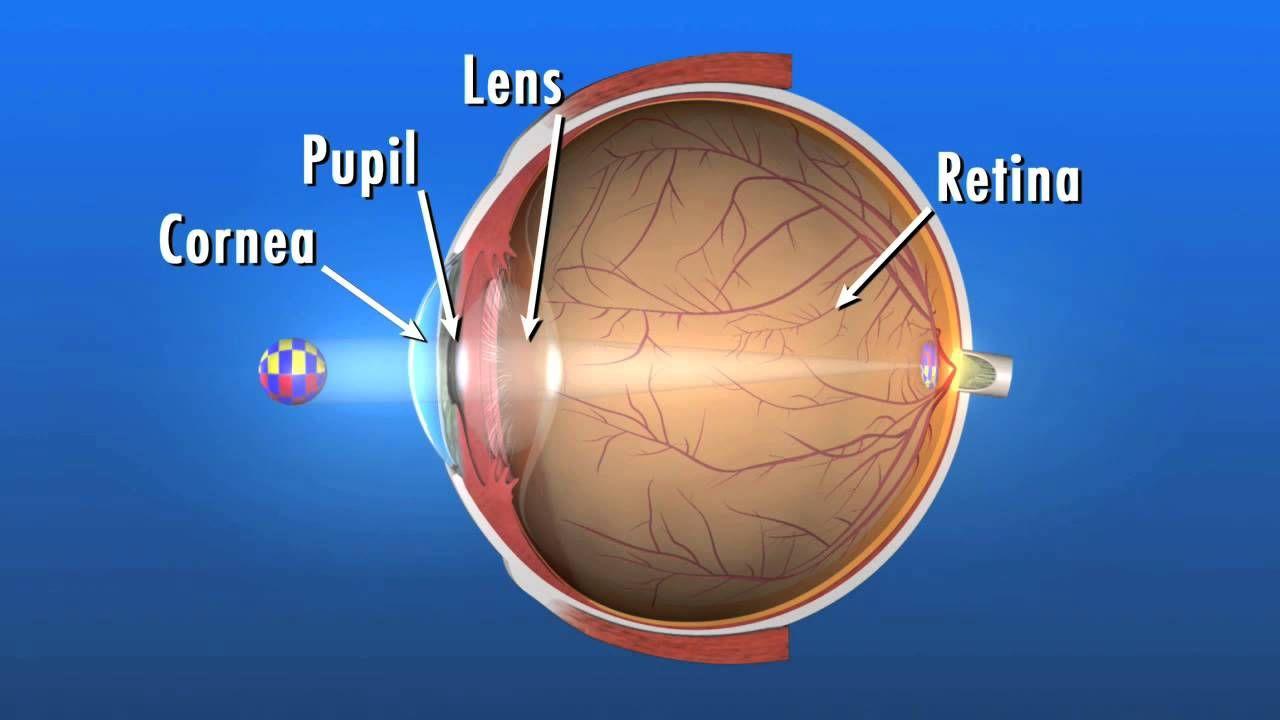
Market Key Trends
One of the key drivers of growth in the global NPHP5 retinal degeneration treatment market is the increasing investment in research and development activities. Pharmaceutical companies, biotechnology firms, academic institutions, and government agencies are actively pursuing innovative approaches to discover and develop novel therapies for NPHP5 retinal degeneration, leveraging advances in genetics, molecular biology, and ocular drug delivery technologies.
Key Takeaways
ProQR Therapeutics, Editas Medicine, Nanoscope Therapeutics, Inc., jCyte, Inc., Biogen, Novartis AG, Spark Therapeutics, MeiraGTx, NightstaRx, Beacon Therapeutics, Applied Genetic Technologies Corporation, ViGeneron and RetinAI Medical
Moreover, the regulatory landscape plays a critical role in shaping Global NPHP5 Retinal Degeneration Treatment Market Demand Regulatory agencies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe oversee the approval and marketing authorization of new therapies, ensuring their safety, efficacy, and quality standards. Expedited pathways, orphan drug designations, and regulatory incentives are available to facilitate the development and approval of treatments for rare diseases like NPHP5 retinal degeneration, providing opportunities for accelerated market entry and commercialization.
Furthermore, market forecasts indicate a promising outlook for the global NPHP5 retinal degeneration treatment market, with steady growth projected in the coming years. Factors such as increasing awareness of genetic disorders, advancements in precision medicine, and rising healthcare expenditure contribute to the expansion of the market. Additionally, the growing pipeline of novel therapies and the potential for breakthrough innovations offer opportunities for market players to capitalize on unmet medical needs and gain a competitive edge in the evolving landscape of NPHP5 retinal degeneration treatment.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- IT, Cloud, Software and Technology


