Pharmaceutical Sterility Testing Market Current Trends, Technology and Industry Analysis 2032
Pharmaceutical sterility testing is a critical process in ensuring the safety and efficacy of pharmaceutical products, especially those administered through injectables, ophthalmic solutions, and other sterile dosage forms. This testing helps confirm that pharmaceutical products are free from viable contaminating microorganisms, such as bacteria, fungi, and yeast, that can compromise their safety. The sterility testing process is mandatory during the manufacturing of drugs, biologics, and medical devices, providing assurance that these products meet the stringent regulatory standards imposed by health authorities like the FDA, EMA, and others.
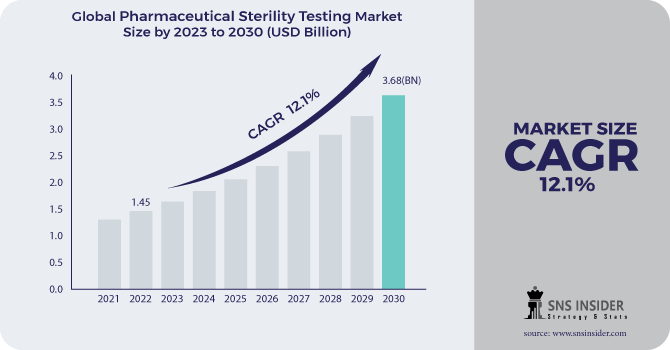
The Pharmaceutical Sterility Testing Market Size was valued at USD 1.50 billion in 2023 and is expected to reach USD 3.84 billion by 2032 and grow at a CAGR of 11.03% over the forecast period 2024-2032.
Future Scope
The future of pharmaceutical sterility testing is set to be shaped by advancements in rapid testing methods and automation technologies. Rapid microbiological methods (RMM) are expected to reduce the time needed to confirm sterility from days to hours, enhancing efficiency in production timelines. With the increasing complexity of biologics and personalized medicines, such as cell and gene therapies, more sophisticated sterility testing techniques will be required to meet the specific demands of these products. Automation, combined with AI-driven data analytics, will also enable better detection capabilities and more reliable sterility assessments.
Trends
One of the key trends in pharmaceutical sterility testing is the move toward rapid and automated testing methods. These advanced systems allow manufacturers to reduce turnaround times and increase production efficiency without compromising safety standards. Another trend is the increasing use of closed and barrier systems, which minimize the risk of contamination during testing and sample handling. As biologic drugs and personalized therapies become more prevalent, sterility testing procedures are being adapted to meet the stringent requirements of these products, with a growing focus on ensuring aseptic conditions throughout the manufacturing process.
Applications
Sterility testing is primarily applied to ensure the safety of injectables, vaccines, biologics, and ophthalmic solutions. It is a critical component in the quality control processes of pharmaceutical companies and is also required for medical devices such as catheters and surgical instruments. In the biotechnology sector, sterility testing is used for the validation of biopharmaceutical products and during clinical trials to ensure the safety of investigational products. Contract research organizations (CROs) and manufacturing facilities also perform sterility testing to comply with regulatory standards before market release.
Key Points
· Pharmaceutical sterility testing is essential for ensuring the safety of sterile drugs and devices.
· Rapid microbiological methods are transforming sterility testing with faster turnaround times.
· Automation and AI-driven systems are improving detection accuracy and efficiency.
· Sterility testing is critical for injectables, vaccines, biologics, and medical devices.
· Regulatory bodies impose stringent requirements on sterility testing protocols.
Conclusion
Pharmaceutical sterility testing remains a cornerstone of ensuring the safety and quality of pharmaceutical products. As the industry shifts toward biologics and personalized medicines, the need for more advanced, rapid, and automated sterility testing techniques will grow. The implementation of these technologies will not only meet regulatory requirements but also improve production efficiencies, ensuring that patients receive safe and effective sterile products. With continuous innovations on the horizon, sterility testing will continue to play a pivotal role in the pharmaceutical and biotechnology sectors.
Read More Details: https://www.snsinsider.com/reports/pharmaceutical-sterility-testing-market-2520
Contact Us:
Akash Anand — Head of Business Development & Strategy
Email: info@snsinsider.com
Phone: +1–415–230–0044 (US) | +91–7798602273 (IND)
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jeux
- Gardening
- Health
- Domicile
- Literature
- Music
- Networking
- Autre
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- IT, Cloud, Software and Technology


