Global Single-use Medical Device Reprocessing Industry: Key Statistics and Insights in 2024-2032
Summary:
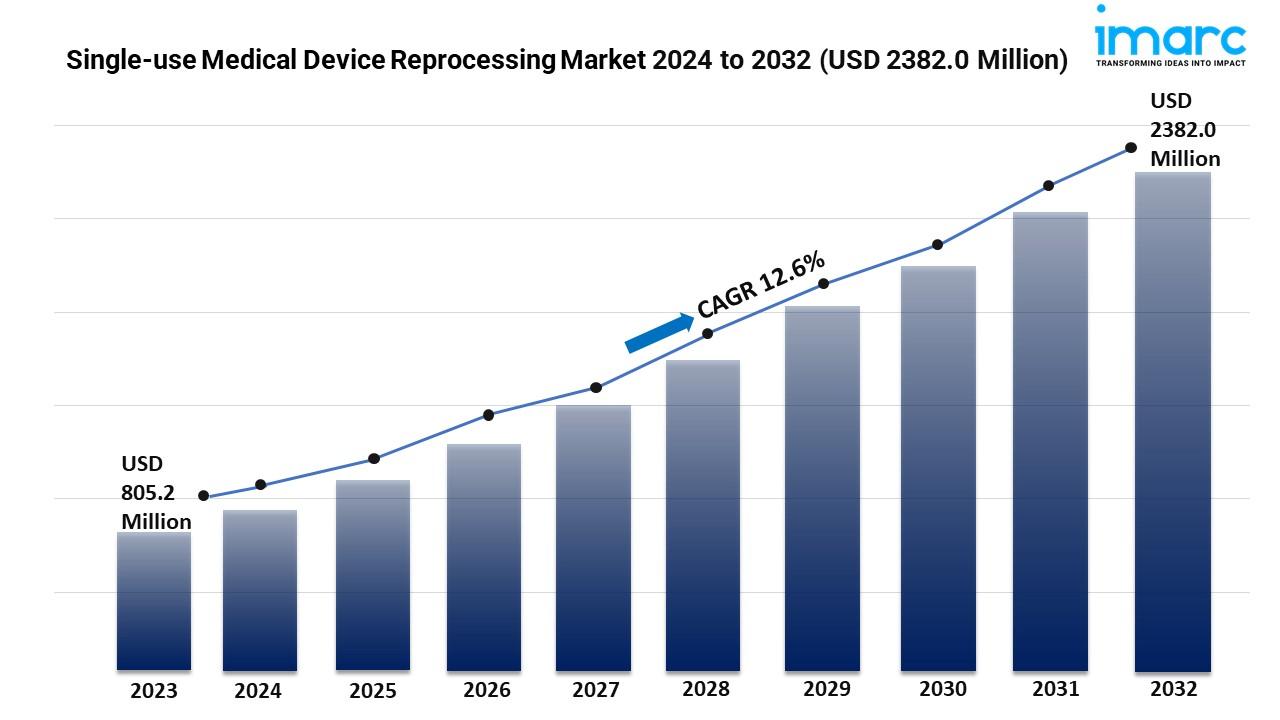
- The global single-use medical device reprocessing market size reached USD 805.2 Million in 2023.
- The market is expected to reach USD 2382.0 Million by 2032, exhibiting a growth rate (CAGR) of 12.6% during 2024-2032.
- North America leads the market, accounting for the largest single-use medical device reprocessing market share.
- Based on the application, the market has been divided into general surgery, anesthesia, arthroscopy and orthopaedic surgery, cardiology, gastroenterology, gynaecology, urology, and others.
- Hospitals account for the majority of the market share owing to the increasing number of patients seeking effective healthcare services.
- The rising need for cost reduction in healthcare services is impelling the growth of the market.
- Environmental sustainability is becoming a priority in various sectors, including healthcare, driving the need for single-use medical devices.
Industry Trends and Drivers:
- Cost Efficiency and Budget Constraints in Healthcare Systems:
The demand for cheaper healthcare is boosting the market. Reprocessing single-use medical devices (SUDs) involves cleaning, disinfecting, and sterilizing them for reuse. This saves healthcare facilities a lot of money. Such savings are vital for staying within budgets. Moreover, reprocessed devices are cheaper than new ones. This enables hospitals and clinics to invest more in patient care or new technologies.
- Environmental Concerns and Sustainability Practices:
Environmental sustainability is now crucial in many sectors, including healthcare. This shift has increased the demand for single-use medical devices. Reprocessing these devices cuts medical waste significantly. This is vital, as healthcare facilities produce a lot of waste each year. By reusing devices, hospitals can reduce their environmental impact. This action lowers non-biodegradable waste in landfills and lessens the need for new materials. Moreover, it supports global efforts to make healthcare more sustainable. This is achieved by managing waste and conserving energy and water in the production and disposal of devices.
- Regulatory Support and Advancements in Reprocessing Technologies:
The market's growth is boosted by supportive regulations from global healthcare authorities. These bodies have established strict guidelines for reprocessing single-use devices (SUDs). This ensures these devices match the safety and performance of new ones. Such regulations are increasing healthcare providers' confidence in using reprocessed devices. Moreover, advancements in reprocessing technology are enhancing the efficiency and safety of these devices. This makes them more attractive to healthcare facilities.
Request for a sample copy of this report: https://www.imarcgroup.com/single-use-medical-device-reprocessing-market/requestsample
Single-use Medical Device Reprocessing Market Report Segmentation:
By Device Type:
- Class I Devices
- Laparoscopic Graspers
- Scalpels
- Tourniquet Cuffs
- Other Class I Devices
- Class II Devices
- Pulse Oximeter Sensors
- Sequential Compression Sleeves
- Catheters and Guidewires
- Other Class II Devices
Class II devices represent the largest segment as they allow healthcare facilities to significantly reduce procurement costs.
By Application:
- General Surgery
- Anesthesia
- Arthroscopy and Orthopaedic Surgery
- Cardiology
- Gastroenterology
- Gynaecology
- Urology
- Others
Based on the application, the market has been divided into general surgery, anesthesia, arthroscopy and orthopaedic surgery, cardiology, gastroenterology, gynaecology, urology, and others.
By End User:

- Hospitals
- Ambulatory Surgical Centers
- Others
Hospitals account for the majority of the market share owing to the increasing number of patients seeking effective healthcare services.
Regional Insights:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America’s dominance in the single-use medical device reprocessing market is attributed to the rising focus on environmental sustainability and waste reduction.
Top Single-use Medical Device Reprocessing Market Leaders:
The single-use medical device reprocessing market research report outlines a detailed analysis of the competitive landscape, offering in-depth profiles of major companies. Some of the key players in the market are:

- Arjo Inc.
- Innovative Health
- Johnson & Johnson
- Medline Industries LP
- NEScientific Inc.
- Steripro Canada
- Stryker Corporation
- SureTek Medical
- Vanguard AG
Note: If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145