Common Misconceptions About CAPA Programs and the Truth Behind Them
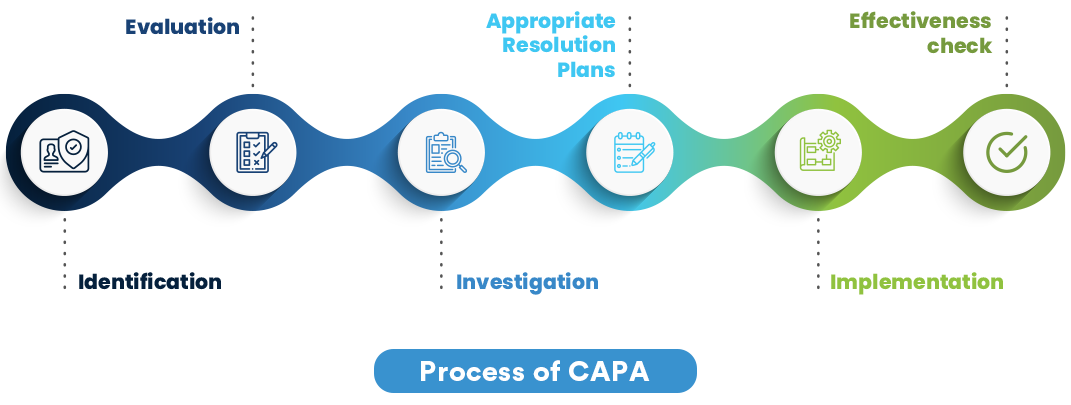
In the ever-evolving landscape of quality management, CAPA programs stand as a cornerstone for ensuring product integrity and regulatory compliance. However, despite their critical importance, several misconceptions cloud their true value and implementation.
Understanding CAPA: More Than Just a Compliance Requirement
CAPA: Beyond Regulatory Mandates
One prevalent misconception is that CAPA is merely a checkbox activity to satisfy regulatory bodies. In reality, CAPA (Corrective and Preventive Actions) is a strategic tool that drives continuous improvement within organizations. While CAPA ISO 9001 and CAPA ISO 13485 frameworks mandate its implementation, the true essence of CAPA lies in its ability to identify root causes, mitigate risks, and prevent recurrence of issues, thereby enhancing overall quality and operational efficiency.
The Strategic Importance of a Robust CAPA Program
Another myth is that CAPA programs are solely reactive, addressing problems after they occur. On the contrary, an effective CAPA program encompasses both corrective and preventive measures, fostering a proactive culture that anticipates potential issues and implements strategies to avert them.
Debunking Myths: CAPA Program Misconceptions
CAPA Programs Are Time-Consuming and Resource-Intensive
Many organizations believe that establishing and maintaining a CAPA program is burdensome and diverts resources from core activities. However, with advanced quality management systems like ComplianceQuest, integrating a CAPA program becomes streamlined and efficient. Automating workflows, facilitating real-time data analysis, and enhancing collaboration can significantly reduce the time and resources required, making CAPA programs both manageable and highly effective.
CAPA Programs Are Only for Large Enterprises
A common misconception is that CAPA programs are exclusively designed for large corporations with extensive resources. In reality, businesses of all sizes, including those with fewer than 250 employees, can greatly benefit from implementing a CAPA program. Tailored solutions can address the unique challenges faced by smaller organizations in the Life Sciences and Manufacturing sectors, ensuring scalability and flexibility without compromising on quality or compliance.
CAPA ISO 9001 and CAPA ISO 13485: Clarifying the Differences
CAPA ISO 9001 vs. CAPA ISO 13485: Understanding the Nuances
While both CAPA ISO 9001 and CAPA ISO 13485 emphasize the importance of corrective and preventive actions, they cater to different industries with distinct requirements. CAPA ISO 9001 focuses on general quality management systems applicable across various sectors, whereas CAPA ISO 13485 is tailored specifically for the Medical Device industry, addressing the stringent regulatory standards and unique challenges inherent to this field.
Implementing CAPA Programs: Industry-Specific Considerations
Implementing a CAPA program requires a nuanced understanding of industry-specific standards. For instance, CAPA ISO 13485 demands a more rigorous approach to risk management and documentation compared to CAPA ISO 9001. Organizations must align their CAPA programs with the specific requirements of their industry to ensure compliance and achieve optimal quality outcomes.
The Role of Technology in Enhancing CAPA Programs
Leveraging Advanced Systems for Effective CAPA Management
A significant misconception is that CAPA programs cannot be effectively managed without extensive manual processes. Modern quality management systems, such as ComplianceQuest, offer sophisticated tools that automate CAPA workflows, track progress, and provide comprehensive analytics.
Integrating CAPA with Other Quality Management Processes
Another myth is that CAPA operates in isolation from other quality management processes. In reality, an integrated approach where CAPA is seamlessly connected with processes like Product Lifecycle Management (PLM), Quality Management Systems (QMS), Supplier Relationship Management (SRM), and Environmental Health and Safety (EHS) can create a cohesive quality ecosystem.
Measuring the Success of CAPA Programs
Key Performance Indicators for CAPA Effectiveness
Measuring the success of CAPA programs involves tracking key performance indicators (KPIs) that reflect the program’s impact on quality and compliance. Metrics such as the number of corrective actions closed within target timelines, recurrence rates of issues, and the effectiveness of preventive measures provide valuable insights into the program’s performance. Utilizing advanced quality management systems facilitates the accurate measurement and analysis of these KPIs, enabling continuous refinement and optimization of CAPA programs.
Continuous Improvement: The Lifeblood of CAPA Programs
The ultimate goal of any CAPA program is to drive continuous improvement within the organization. By regularly evaluating the effectiveness of corrective and preventive actions, organizations can identify areas for enhancement and implement strategies to elevate their quality management practices. This commitment to ongoing improvement not only ensures sustained compliance with standards like CAPA ISO 9001 and CAPA ISO 13485 but also positions organizations to thrive in competitive and highly regulated industries.
The Human Factor: Building a Culture of Quality Through CAPA
Fostering Employee Engagement in CAPA Processes
A misconception exists that CAPA programs are solely the responsibility of the Quality Assurance team. In reality, fostering a culture of quality requires active participation from all employees. Engaging staff at all levels in CAPA processes encourages ownership, accountability, and a collective commitment to continuous improvement. This holistic approach not only enhances the effectiveness of CAPA programs but also drives overall organizational excellence.
Training and Education: Key Components of an Effective CAPA Program
Effective CAPA programs are underpinned by comprehensive training and education initiatives. Ensuring that employees understand the principles and practices of CAPA, as well as their roles in the process, is crucial for successful implementation. Regular training sessions and ongoing education help maintain a high level of competency and awareness, enabling organizations to navigate the complexities of quality management with confidence and proficiency.
Conclusion
In the competitive and highly regulated environments of the Life Sciences and Manufacturing sectors, effective CAPA programs are indispensable for ensuring quality, compliance, and continuous improvement. ComplianceQuest stands out as an essential tool for businesses in 2025, offering a comprehensive and advanced quality management system that seamlessly integrates CAPA processes with other critical functions like PLM, QMS, SRM, and EHS. By leveraging ComplianceQuest, organizations can overcome common misconceptions about CAPA programs, implement robust and scalable solutions, and stay ahead in an increasingly demanding market.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- IT, Cloud, Software and Technology


