Antibodies Contract Manufacturing Market Size, Growth Outlook 2035
Market Overview
The Antibodies Contract Manufacturing Market is experiencing robust growth, driven by the rising demand for monoclonal antibodies (mAbs), therapeutic antibodies, and biosimilars. Contract manufacturing organizations (CMOs) provide specialized services for antibody production, including cell line development, upstream and downstream processing, purification, and fill-finish services. The increasing prevalence of chronic diseases such as cancer, autoimmune disorders, and infectious diseases, along with the growing adoption of biopharmaceutical outsourcing, is fueling market expansion.
Market Size and Share
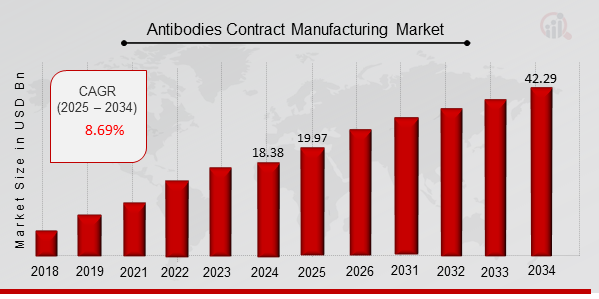
The global Antibodies Contract Manufacturing Market Size was estimated at 18.38 (USD Billion) in 2024. The Antibodies Contract Manufacturing Market Industry is expected to grow from 19.97 (USD Billion) in 2025 to 42.29 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 8.69% during the forecast period (2025 - 2034). North America dominates the market due to the presence of leading biopharmaceutical companies, advanced bioprocessing technologies, and strong regulatory frameworks. The Asia-Pacific contract antibody production market is witnessing rapid growth due to increasing investments in biologics manufacturing facilities and the availability of cost-effective contract manufacturing services.
Market Drivers
- Rising Demand for Monoclonal Antibodies: The growing application of mAbs in oncology, immunology, and infectious diseases is boosting demand for contract antibody production.
- Growing Outsourcing Trends in Biopharmaceuticals: Companies are increasingly outsourcing antibody manufacturing services to CMOs to reduce production costs and focus on core R&D.
- Advancements in Bioprocessing Technologies: Innovations in single-use bioreactors, cell culture optimization, and chromatography techniques are enhancing efficiency in antibody production.
- Expanding Biosimilars Market: The increasing development of biosimilar antibodies is creating new opportunities for contract biologics manufacturing.
Challenges and Restraints
- High Costs Associated with Large-Scale Antibody Production: The cost-intensive nature of biopharmaceutical manufacturing poses challenges for smaller companies.
- Regulatory Compliance and Quality Control Issues: Strict guidelines from the FDA, EMA, and other regulatory agencies necessitate rigorous quality control in biologics contract manufacturing.
- Limited Availability of Skilled Workforce: The shortage of bioprocessing experts can hinder market growth.
Market Trends
- Adoption of Single-Use Bioprocessing Technologies: The shift towards disposable bioprocessing systems is improving flexibility and reducing contamination risks in contract antibody production.
- Increasing Focus on Antibody-Drug Conjugates (ADCs): Contract manufacturers are expanding their capabilities to support the rising demand for ADCs in targeted cancer therapies.
- Strategic Collaborations Between Biotech Firms and CMOs: Pharmaceutical companies are forming alliances with biologics CMOs to enhance antibody therapeutic production capabilities.
Regional Analysis
- North America: The dominant region due to strong biopharmaceutical infrastructure, high investment in biologics manufacturing, and the presence of major CMOs.
- Europe: Significant growth driven by increasing adoption of biosimilar contract manufacturing and stringent regulatory frameworks.
- Asia-Pacific: Fastest-growing market, with countries like China, India, and South Korea emerging as key hubs for antibody contract manufacturing services.
- Rest of the World: Moderate market expansion, with increasing interest in Latin America and the Middle East.
Segmental Analysis
- By Product Type:
- Monoclonal Antibodies (mAbs)
- Polyclonal Antibodies
- Antibody Fragments
- Antibody-Drug Conjugates (ADCs)
- By Service Type:
- Cell Line Development & Optimization
- Process Development & Scale-Up
- Upstream & Downstream Processing
- Analytical & Quality Control Services
- Fill-Finish & Packaging
- By End-User:
- Biopharmaceutical Companies
- Academic & Research Institutes
- Contract Research Organizations (CROs)
Key Market Players
· Charles River Laboratories
· AbCellera
· AGC Biologics
· Novavax
· Lonza
· MilliporeSigma
· ProBioGen AG
Recent Developments
- Expansion of Biologics Manufacturing Facilities: Leading CMOs are investing in new large-scale antibody production plants.
- Launch of AI-Powered Bioprocessing Solutions: AI-driven process optimization platforms are enhancing efficiency in contract antibody production.
- Strategic Mergers and Acquisitions: Companies are acquiring smaller biologics contract manufacturers to expand capabilities.
For more information, please visit us at marketresearchfuture
- Antibodies_Contract_Manufacturing_Market_Size
- Antibodies_Contract_Manufacturing_Market_Share
- Antibodies_Contract_Manufacturing_Market_Growth
- Antibodies_Contract_Manufacturing_Market_Analysis
- Antibodies_Contract_Manufacturing_Market_Trends
- Antibodies_Contract_Manufacturing_Market_Forecast
- Antibodies_Contract_Manufacturing_Market_Segments
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- الألعاب
- Gardening
- Health
- الرئيسية
- Literature
- Music
- Networking
- أخرى
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- IT, Cloud, Software and Technology


