Pharmacovigilance Market: Emerging Technologies and Trends 2024-2032
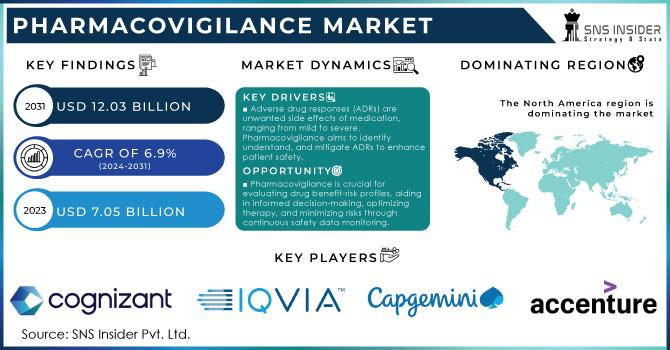
The global pharmacovigilance market, dedicated to the detection, assessment, understanding, and prevention of adverse drug reactions, is experiencing substantial growth. Valued at USD 7.20 billion in 2023, the market is projected to reach USD 18.52 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 11.09% over the forecast period from 2024 to 2032.
Regional Analysis
North America currently leads the pharmacovigilance market, holding a significant share due to stringent regulatory frameworks and a robust pharmaceutical industry. Europe follows closely, driven by comprehensive drug safety regulations. The Asia-Pacific region is anticipated to witness the fastest growth, attributed to increasing clinical trials, rising healthcare infrastructure investments, and growing awareness about drug safety.
Get Free Sample Report @ https://www.snsinsider.com/sample-request/3095
Market Segmentation
The pharmacovigilance market is segmented based on several key factors:
- Product Life Cycle Phases: Including Phase III and Phase IV (post-marketing) clinical trials.
- Service Providers: Encompassing in-house departments and contract outsourcing organizations.
- Type: Covering spontaneous reporting, targeted spontaneous reporting, and others.
- Process Flow: Involving case data collection, signal detection, risk assessment, and more.
- Therapeutic Areas: Such as oncology, neurology, cardiology, and others.
- End-Use: Including pharmaceutical companies, contract research organizations (CROs), and other healthcare entities.
Key Players:
ArisGlobal, Cognizant, IBM, Clinquest Group B.V. (Linical Americas), Accenture, Laboratory Corporation of America Holdings, IQVIA,Capgemini, ICON plc., ITClinical, ClinChoice (formerly FMD K&L), TAKE Solutions Limited, Wipro, Parexel International (MA) Corporation, BioClinica Inc. (Clario), United BioSource LLC and others.
Key Highlights
- The rising incidence of adverse drug reactions (ADRs) necessitates enhanced pharmacovigilance practices.
- Increasing complexity in drug regimens for chronic diseases like cancer and diabetes drives demand for robust safety monitoring.
- The expansion of drug development pipelines, including personalized medicines and biosimilars, underscores the need for stringent safety protocols.
- The shift towards decentralized and adaptive clinical trials presents new challenges and opportunities for comprehensive pharmacovigilance services.
Future Outlook
The pharmacovigilance landscape is set to evolve with the integration of advanced technologies such as big data analytics, artificial intelligence (AI), and cloud computing. These innovations aim to enhance data accuracy, streamline safety assessments, and improve overall drug monitoring processes. Collaborations between major industry players, exemplified by partnerships like that of IQVIA and Alibaba Cloud to deploy clinical solutions in China, highlight a trend towards leveraging technological advancements to bolster pharmacovigilance efforts.
Conclusion
As the global pharmaceutical industry continues to grow, the importance of effective pharmacovigilance cannot be overstated. Ensuring drug safety through meticulous monitoring and evaluation not only protects patient health but also enhances the credibility of healthcare systems worldwide. The projected growth of the pharmacovigilance market reflects a collective commitment to upholding the highest standards of drug safety and efficacy.
Contact Us:
Jagney Dave - Vice President of Client Engagement
Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- IT, Cloud, Software and Technology


