Aluminum chloride has many uses
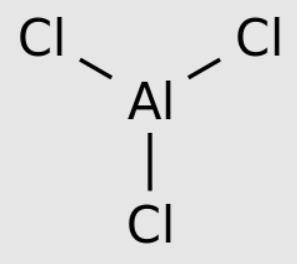
Aluminum chloride is a compound, an aluminum salt, with the molecular formula AlCl3. Each aluminum chloride unit has one aluminum (Al) atom and three chlorine (Cl) atoms. Therefore, it is also known as aluminum trichloride. The purest solid form of aluminum chloride is a white powder. When it is contaminated with iron, it displays yellow. Aluminum chloride can also exist in the form of liquid and gas. Its melting and boiling points are very low.
Solid aluminum chloride
In 1825, Danish physicist and chemist Hans Christian Oersted first discovered aluminum chloride. It is one of the oldest chemical substances in organic chemistry.
Aluminum chloride usage
Aluminum chloride has many uses, mainly in the manufacturing industry. One method of producing pure aluminum metal is to use aluminum chloride in specific chemical reactions/processes to ultimately obtain pure aluminum. In addition, AlCl3 is widely used in the production of rubber, lubricants, wood preservatives, pesticides and paints.
Aluminum chloride can also be found in today's pharmaceuticals. A common drug application is an anti bleeding agent. Dentists often use compounds containing aluminum chloride to prevent excessive bleeding after tooth extraction. Aluminum chloride exerts a hemostatic effect by reacting with the blood, thereby preventing chemicals from entering the patient's system.
Frey's syndrome is a syndrome that often occurs after parotid gland surgery. This situation occurs when the nerves that previously supported the salivary glands now dominate the sweat glands in the facial area. The result of this incorrect nerve regeneration is that a person experiences excessive sweating related to food. This includes eating, smelling, or thinking about food. Any activity that can cause normal individuals to secrete saliva can lead to excessive facial sweating in patients with Freund's syndrome. There are several treatment methods for this situation. Local aluminum chloride, due to its sweating properties, is one of the popular non-surgical options for treating this condition.
Aluminum chloride deodorant
The use of aluminum chloride has affected the daily lives of most of us, as it exists in antiperspirants and deodorants. The aluminum chloride in antiperspirant works because this chemical will produce gel when interacting with sweat. This gel forms a non permanent barrier at the skin level to prevent sweat from flowing out through the pores of the skin. In addition, the presence of aluminum chloride in antiperspirants and deodorants can cause skin pores to contract and close, once again limiting the amount of water that can reach the skin.
If there is no prescription, the aluminum chloride content in antiperspirants and deodorants is 15%. However, for those who sweat excessively, some prescription grade products contain up to 20% aluminum chloride.
When pure aluminum chloride comes into direct contact with the skin, the skin may experience burning, tingling, tingling, and itching. The daily use of antiperspirants and deodorants will not cause this reaction, as the aluminum chloride in this product combines with other compounds. Although the use of antiperspirants can cause some tingling and discomfort if the skin is cut or scratched by a razor.
Aluminum chloride hazards
Aluminum chloride is widely used in the manufacturing industry in both liquid and solid forms. In its purest state, it is a highly corrosive chemical substance. The severity of any exposure directly depends on the concentration and duration of the exposure. The following is an overview of the reaction types of AlCl3 in contact with humans.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Giochi
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Altre informazioni
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- IT, Cloud, Software and Technology


