Alzheimer’s disease (AD) is a progressive neurodegenerative disease with an insidious onset. Clinically, it is characterized by comprehensive dementia manifestations such as memory impairment, aphasia, apraxia, agnosia, impairment of visuospatial skills, executive dysfunction, and personality and behavioral changes. Alzheimer’s disease is progressive and irreversible and can only be delayed with medication. Once it develops into moderate or severe disease, it will cause significant harm to the lives of patients and their families.
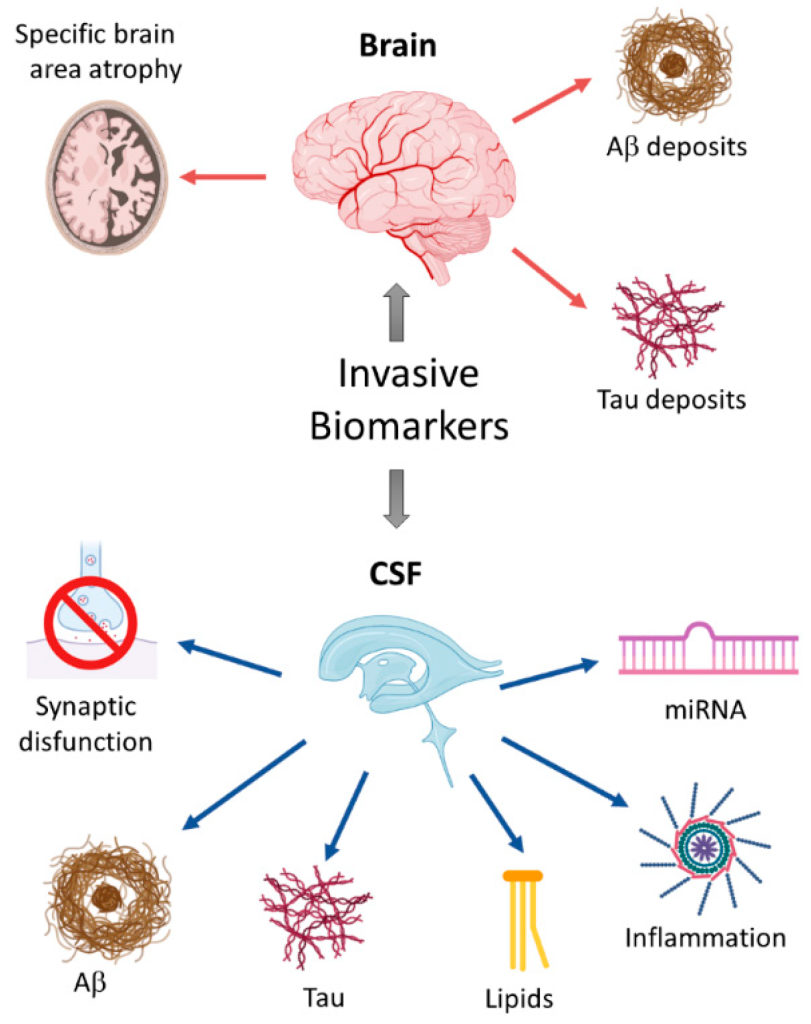
Figure 1. Schematic overview of invasive biomarkers.
Pathological Mechanism of AD
The pathological mechanism of AD is currently unclear, and is currently summarized as neurodegenerative changes and inflammatory response. Neurodegenerative changes involve neuronal degeneration, nerve cell apoptosis, and the generation of new neurons. Inflammatory response refers to the inflammatory response in the brain of AD patients, including the release of activated cytokines and inflammatory cytokines. In addition, many studies have shown that the pathological mechanism of AD is also affected by protein oxidative stress. Oxidative stress refers to the production of free radicals and the increase in reactive oxygen species under conditions of intracellular hypoxia or anoxia, leading to the destruction of cell structure and function. Studies have found that oxidative stress can affect the apoptosis and degeneration of nerve cells in the brains of AD patients, thus increasing the severity of the disease. There are reports that AD is also affected by neurotransmitters. The level of neurotransmitters in the brains of AD patients is lower than that of normal people. This may be one of the reasons for the cognitive impairment of AD patients. In addition, AD patients have obvious amyloidosis in their brains. Amyloidosis refers to a variety of pathological changes that occur in the brains of AD patients, including amyloid crystals, neuronal degeneration, and nerve cell apoptosis. Such lesions may be a key cause of AD and serve as diagnostic markers for AD.
AD Diagnostic Markers
Apolipoprotein Eε4
Apolipoprotein Eε4 (APOEε4) is a very clear risk gene for late-onset AD, which may advance the age of onset and accelerate the development of cognitive decline. It has been recognized as a genetic marker of AD. ApoE is a key component of plasma lipoproteins, which can produce β-amyloid peptide and maintain neurological function.
Under the action of hydrolyzing amyloid precursor protein (APP), β⁃ and γ⁃secretase can produce β⁃amyloid protein (Aβ). Such polypeptides are distributed in many cells and can also be found in blood, cerebrospinal fluid (CSF) and brain interstitial fluid. The most widespread subtypes of Aβ are Aβ40 and 42. The latter are neurotoxic and are related to the formation of AD. Most of them are generated from microglia and astrocytes, have hydrophobic characteristics, and play a major role in plaque formation. Aβ42 in CSF is generally considered to be a biomarker of AD and is helpful in diagnosing preclinical AD. Lower levels of Aβ42 in the cerebrospinal fluid are associated with higher Aβ plaques in the brain, and the concentration levels of Aβ42 in the cerebrospinal fluid of AD patients are significantly reduced compared with normal controls. Currently, the detection of Aβ42 in cerebrospinal fluid can be carried out using immunological technology or mass spectrometry. However, the specificity of Aβ42 in detecting AD needs to be improved. Some studies have shown that the ratio of Aβ42/Aβ40 can be used to judge amyloid deposition in the brain in the early stages of the disease. The specificity of this method is significantly better than that of measuring Aβ42 alone. However, the detection of cerebrospinal fluid is invasive and is not conducive to early screening. Currently, more and more scholars are beginning to explore blood biomarkers. Aβ protein can be measured in plasma, but the Aβ concentration in plasma will be affected by factors such as platelets, and its correlation with AD is not as good as the Aβ concentration in cerebrospinal fluid. However, gratifyingly, studies have shown that using mass spectrometry to detect plasma Aβ concentration can show a significant correlation with cerebral amyloidosis. Therefore, exploring more effective measurement technologies is the core of breakthroughs in blood screening.
The microtubule system is a component of the neuronal cytoskeleton, and its composition contains tubulin and microtubule-related proteins, with the latter having a higher content of Tau protein. The main function of Tau protein is to bind tubulin and promote the production of microtubules. If Tau is over-phosphorylated, it can accumulate in cells, damage the function of microtubules, and damage the cytoskeleton or axonal transport of nerve cells, thus triggering neurodegeneration. It is generally believed that total⁃t tau (T⁃t tau) and phosphorylated⁃Tau (P⁃tau) concentrations are related to AD. The increase in T-tau reflects the degree of degeneration and damage of neurons and axons. High levels of P-tau suggest the formation of neurofibrillary tangles. CSF P-tau is regarded as the most specific marker of AD, and the concentration of CSF P-tau in AD patients will be significantly increased. Studies have shown that P-tau protein can be used to judge the conversion of MCI to AD, and therefore can be used as a key marker for early clinical diagnosis. Nowadays, P-tau levels in CSF can be measured through immunological methods such as ELISA.
Neurofilament lightweight peptide (NFL)
NFL is a peptide encoded by the NEFL gene. It is an intrinsic cytoskeletal protein that can be used as a biomarker of axonal injury and can be obtained in both cerebrospinal fluid and plasma. Neurons in the brain interstitium and cerebrospinal fluid release NFL after death, and then enter the blood. The serum or plasma level of NFL is closely related to CSF NFL, and is increased in both familial and sporadic AD. In familial AD, patients show increased concentrations of NFL 10 years before the onset of significant clinical symptoms. . Therefore, plasma or serum NFL may be a reliable blood biomarker for neurodegenerative diseases in AD and other neurodegenerative diseases.
Plasma glial fibrillary acidic protein (GFAP)
GFAP is a key indicator used to monitor astrocyte viability. Previous studies have shown that GFAP is significantly increased in the brain and CSF of AD patients, suggesting that GFAP may become a potential biomarker of AD. Studies have shown that serum GFAP levels in AD patients are significantly higher than those in normal controls. In addition, reports also suggest that the plasma concentration of early-onset AD patients is significantly higher than that of normal controls. While the increase in GFAP concentration in the plasma of late-onset AD patients is not as significant as that of early-onset AD, it is also significantly higher than that of normal controls group. Therefore, GFAP is expected to become a member of the blood AD markers and provide new clues for the clinical diagnosis of AD.
Neurogranin (Ng)
Ng is also a potential biomarker for early diagnosis of AD and is usually expressed in granular structures in pyramidal cells in the hippocampus and cortex. Ng has been shown to be related to synaptic plasticity, synaptic regeneration and other functions mediated by the calcium/calmodulin signaling pathway. Some scholars have compared the levels of neurogranin in the cerebrospinal fluid of patients with AD, frontotemporal dementia, Lewy body dementia, Parkinson’s disease, etc. The results show that the concentration of neurogranin in the cerebrospinal fluid of AD patients is significantly higher than that of other diseases, further illustrating that Higher Ng protein concentration in cerebrospinal fluid has a specific screening effect on AD.


