CAR T Cell Therapy: A Promising New Approach for Cancer Treatment
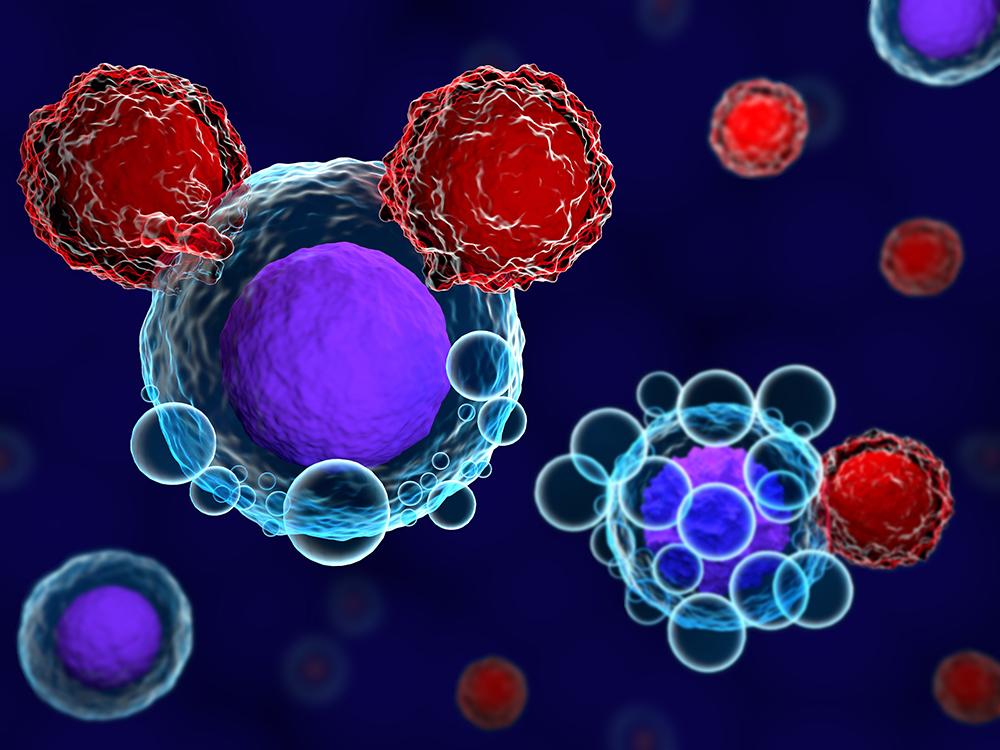
What are CAR T Cells?
Chimeric antigen receptor (CAR) T cell therapy is an immunotherapy that uses engineered immune cells called CAR T cells to fight cancer. In CAR T cell therapy, a patient's own T cells are collected from their blood and engineered to recognize and kill cancer cells by genetically modifying the T cells to express a chimeric antigen receptor (CAR) on their surface. The CAR binds to a specific protein, called an antigen, on the surface of a patient's cancer cells leading the CAR T cells to attack and kill the cancer cells.
How are CAR T Cells Engineered?
To engineer CAR T cells, immune cells called T cells are first collected from a patient's blood through a process called leukapheresis. In the laboratory, a gene is inserted into the T cells that includes instructions for producing a chimeric antigen receptor (CAR). The CAR incorporates three important components - an antigen recognition domain derived from an antibody that binds to a specific antigen on the cancer cell surface, a co-stimulatory domain that activates CAR T cells once engaged, and an activation domain that triggers the full activation of the T cell. Once reprogrammed with the CAR, the engineered CAR T cells can recognize and bind directly to the targeted antigen on cancer cells and activate themselves to kill the cancer cell. The engineered CAR T cells are then expanded in number before being infused back into the patient to target the antigen on their cancer cells.
Types
The first generation CAR T Cell Therapies consisted of only the antigen recognition domain and activation domain. These cells could only attack cancer cells but did not further expand and propagate in the body. Second generation CAR T cells were engineered with an additional co-stimulatory domain that activates not just the T cell but also drives T cell proliferation and persistence. Third generation CAR T cells contain two co-stimulatory domains which further enhance T cell expansion and anti-tumor activity. Different CAR T cell therapies target different antigens like CD19, CD20, HER2, EGFR and CD133 that are expressed on various types of cancer cells. CD19-targeted CAR T cells have shown promising results in B cell malignancies like leukemia and lymphoma. CD20 and HER2-targeted CAR T cells are also being explored for other blood cancers and solid tumors. Ongoing research aims to engineer safer and more effective therapies.
Challenges
While CAR T cell therapy has achieved remarkable responses in certain blood cancers, there are still challenges to address. Toxicities, also known as cytokine release syndrome (CRS), can occur when large numbers of T cells are activated and release inflammatory signaling molecules causing fevers, low blood pressure and cardiac issues in some cases. CRS is usually treated with cytokines that block inflammation. Another major challenge, especially for solid tumors, is the immunosuppressive tumor microenvironment that can inhibit T cell function and persistence in the body.Engineering CAR T cells resistant to suppression or combining CAR T cells with other agents that modify the tumor microenvironment may help address this challenge. Difficulty in homogeneously targeting antigens in solid tumors also limits efficacy. Ongoing innovation in CAR design, targeting multiple antigens, and combination therapy approaches aim to improve outcomes for solid tumors. Long-term persistence of CAR T cells is also a question and developing strategies for repeated CAR T cell infusions or memory T cell formation may help sustain response duration. Managing costs also needs to be addressed as CAR T cell therapy is currently very expensive requiring sophisticated cell production facilities and infrastructure support.
Get More Insights on CAR T Cell Therapy
About Author:
Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Spiele
- Gardening
- Health
- Startseite
- Literature
- Music
- Networking
- Andere
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- IT, Cloud, Software and Technology


