Ebola Vaccine: Making Strides Forward in the Race to Create an Effective Ebola Vaccination Recent Advancements
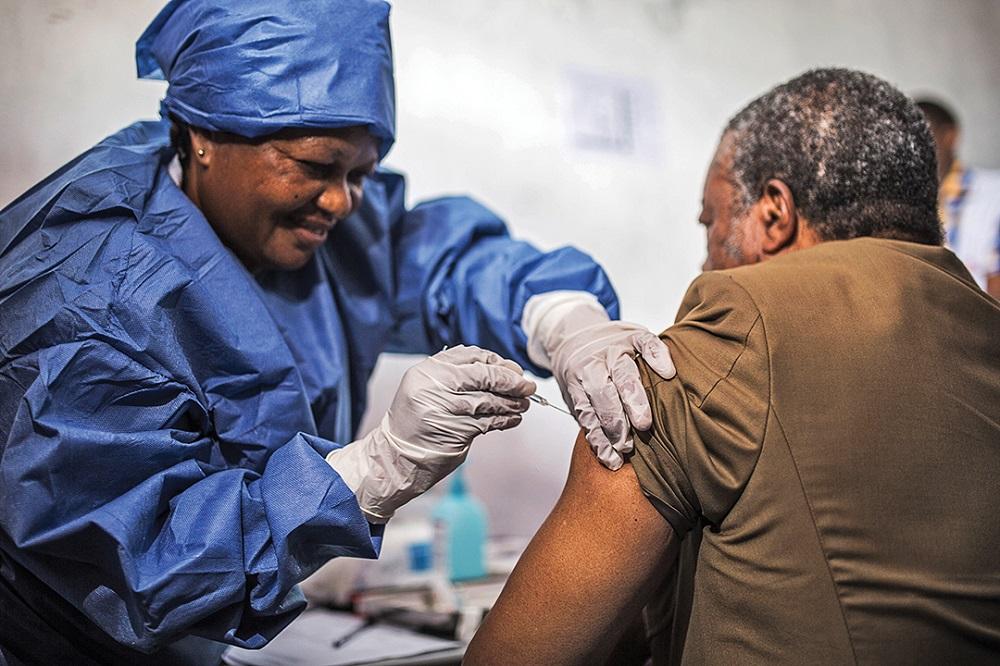
The Ebola virus was first discovered in 1976 during simultaneous outbreaks in what is now the Democratic Republic of the Congo and South Sudan. Since then, periodic outbreaks have occurred across central Africa. The 2013-2016 West African epidemic brought widespread international attention as it caused over 28,000 cases and 11,000 deaths. This outbreak demonstrated the need for a licensed Ebola Vaccine to help control future epidemics. Several vaccines had been in development since the 1990s but none had completed clinical trials at the start of the West African outbreak.
Early Vaccine Candidates
Some of the earliest vaccine candidates included live attenuated and inactivated virus approaches. A live attenuated vaccine called the Zaire ebolavirus vaccine (ZEBOV) was tested in primates in 1996 and conferred full protection against infection. However, concerns over potential reversion to virulence hindered its progression. Inactivated virus vaccines were also studied but required high antigen doses and multiple immunizations to achieve protection in primates. DNA vaccines were another early approach using plasmid DNA to express Ebola viral proteins and induce immune responses. While stimulating antibody and cellular immunity, DNA vaccines provided inconsistent protection in primate studies.
Get more insights on - Ebola Vaccine
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- IT, Cloud, Software and Technology


