Summary:
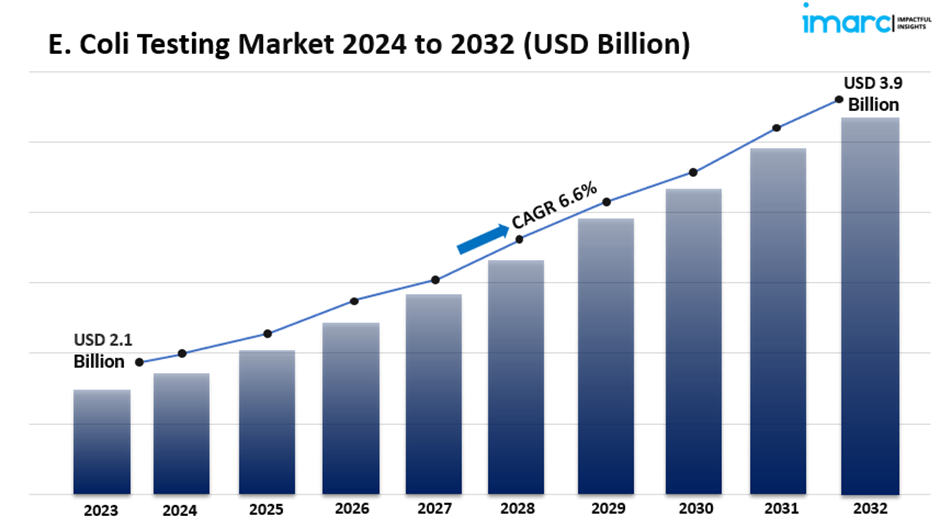
· The global e. coli testing market size reached USD 2.1 Billion in 2023.
· The market is expected to reach USD 3.9 Billion by 2032, exhibiting a growth rate (CAGR) of 6.6% during 2024-2032.
· Based on region, the market has been categorized into North America (United States and Canada); Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others); Europe (Germany, France, United Kingdom, Italy, Spain, Russia, and others); Latin America (Brazil, Mexico, and others); and Middle East and Africa.
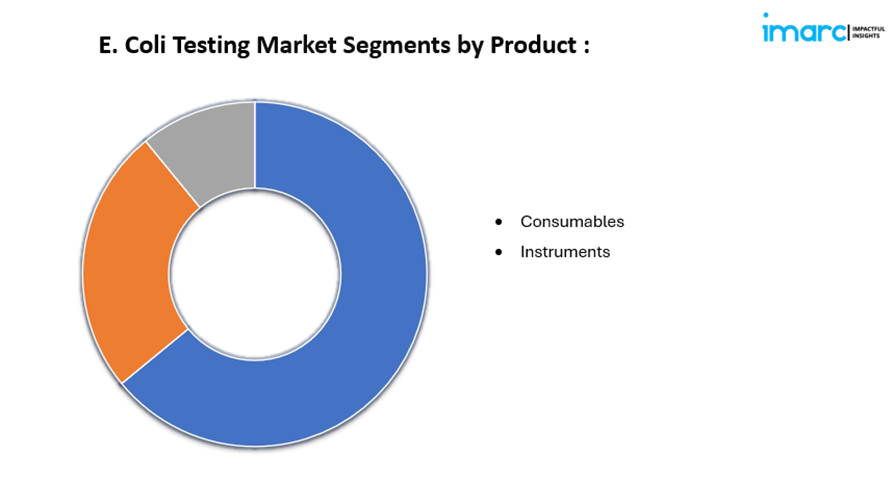
· Based on the product, the market has been divided into consumables and instruments.
· Based on test type, the market has been classified into environmental test (membrane filtration (MF), multiple tube fermentation (MTF), and enzyme substrate methods) and clinical test (polymerase chain reaction (PCR) tests, enzyme immunoassays (EIA), and others).
· Based on the end user, the market has been divided into hospitals and clinics, diagnostic laboratories, and others.
· The rising food safety concerns due to increasing prevalence of food borne diseases is a primary driver of the e. coli testing market.
· The implementation of stringent regulatory standards and development of advanced testing methods are further reshaping the e. coli testing market.
Request to Get the Sample Report:
https://www.imarcgroup.com/e-coli-testing-market/requestsample
Industry Trends and Drivers:
- Growing Food Security Concerns:
Rising food safety concerns are a significant driver of the E. coli testing market, as foodborne illnesses continue to pose substantial risks to public health. E. coli, particularly pathogenic strains, can cause severe gastrointestinal illnesses and potentially lead to life-threatening conditions. High-profile outbreaks linked to contaminated food products have heightened awareness and urgency around food safety, prompting both regulatory bodies and consumers to demand more rigorous testing procedures. The food industry, including producers, processors, and distributors, is under increasing pressure to ensure that their products are free from contaminants, and E. coli testing is a critical component of this assurance. Stringent regulations and standards enforced by various agencies mandate regular testing of food products to detect and mitigate contamination risks.
- Stringent Regulatory Standards:
Stringent regulatory standards are a key factor driving the growth of the E. coli testing market, as governments and regulatory agencies implement rigorous requirements for ensuring food safety and water quality. These regulations are designed to protect public health by mandating regular and accurate testing for pathogens like E. coli in food products, drinking water, and recreational waters. Government agencies and the World Health Organization (WHO) have established comprehensive guidelines and maximum allowable limits for E. coli contamination. Compliance with these regulations is essential for businesses in the food and beverage (F&B) industry, as well as for water treatment facilities, to avoid penalties, product recalls, and legal liabilities. The increasing complexity of regulatory frameworks, coupled with the need to keep up with evolving standards, drives the adoption of advanced E. coli testing methods.
- Advancements in Texting Technologies:
Advancements in testing technologies are significantly impacting the E. coli testing market, as they enhance the speed, accuracy, and reliability of detection methods. Traditional E. coli testing methods, such as culture-based techniques, can be time-consuming and labor-intensive, often requiring several days to obtain results. However, recent technological innovations have introduced rapid diagnostic tools and molecular techniques that offer faster and more precise detection of E. coli. For example, polymerase chain reaction (PCR) technology allows for the amplification and detection of specific genetic material from E. coli, providing results within hours rather than days. Furthermore, the development of portable and automated testing systems further enhances the efficiency of E. coli testing by allowing for on-site and real-time analysis.
E. Coli Testing Market Report Segmentation:
Breakup By Product:
· Consumables
· Instruments
Based on the product, the market has been bifurcated into consumables and instruments.
Breakup By Test Type:
Environmental Test
· Membrane Filtration (MF)
· Multiple Tube Fermentation (MTF)
· Enzyme Substrate Methods
Clinical Test
· Polymerase Chain Reaction (PCR) Tests
· Enzyme Immunoassays (EIA)
· Others
Based on test type, the market has been divided into environmental test (membrane filtration (MF), multiple tube fermentation (MTF), and enzyme substrate methods) and clinical test (polymerase chain reaction (PCR) tests, enzyme immunoassays (EIA), and others).
Breakup By End User:
· Hospitals and Clinics
· Diagnostic Laboratories
· Others
Based on the end user, the market has been classified into hospitals and clinics, diagnostic laboratories, and others.
Breakup By Region:
· North America
· Asia Pacific
· Europe
· Latin America
· Middle East and Africa
Region-wise the market has been segmented into North America (United States and Canada); Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others); Europe (Germany, France, United Kingdom, Italy, Spain, Russia, and others); Latin America (Brazil, Mexico, and others); and Middle East and Africa.
Top E. Coli Testing Market Leaders:
The global e. coli testing market research report outlines a detailed analysis of the competitive landscape, offering in-depth profiles of major companies.
Some of the key players in the market are:
· Accugen Laboratories Inc.
· Alere Inc. (Abbott Laboratories)
· BD (Becton Dickinson and Company)
· bioMérieux (INSTITUT MERIEUX)
· Bio-RAD Laboratories Inc.
· Enzo Life Sciences Inc. (Enzo Biochem Inc.)
· Idexx Laboratories Inc.
· Johnson & Johnson
· Meridian Bioscience Inc.
· Nanologix Inc.
· Pro-Lab Diagnostics
· Qiagen N.V.
· Thermo Fisher Scientific Inc.
Ask Analyst for Customized Report:
https://www.imarcgroup.com/request?type=report&id=2368&flag=C
Key Highlights of the Report:
· Market Performance (2018-2023)
· Market Outlook (2024-2032)
· Market Trends
· Market Drivers and Success Factors
· Impact of COVID-19
· Value Chain Analysis
If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services.
IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St
Brooklyn, NY 11249, USA
Website: imarcgroup.com
Email: sales@imarcgroup.com
Americas: +1-631-791-1145 | Europe & Africa: +44-753-713-2163 | Asia: +91-120-433-0800



