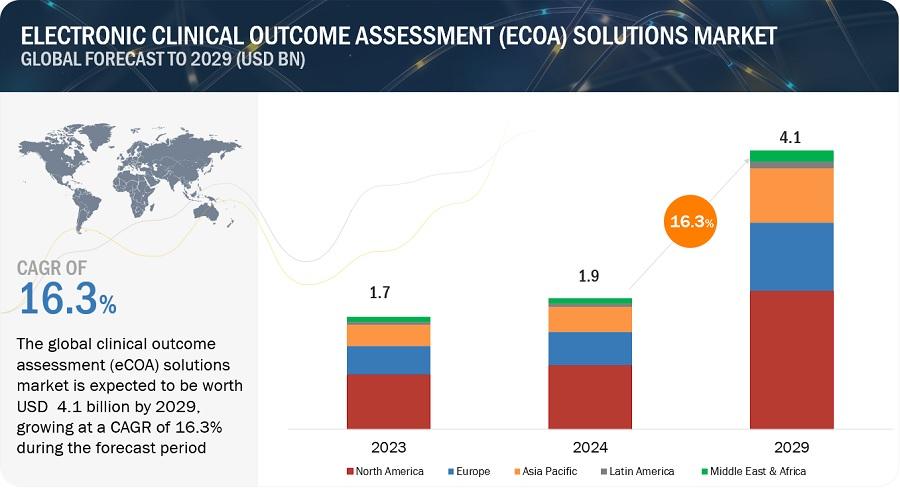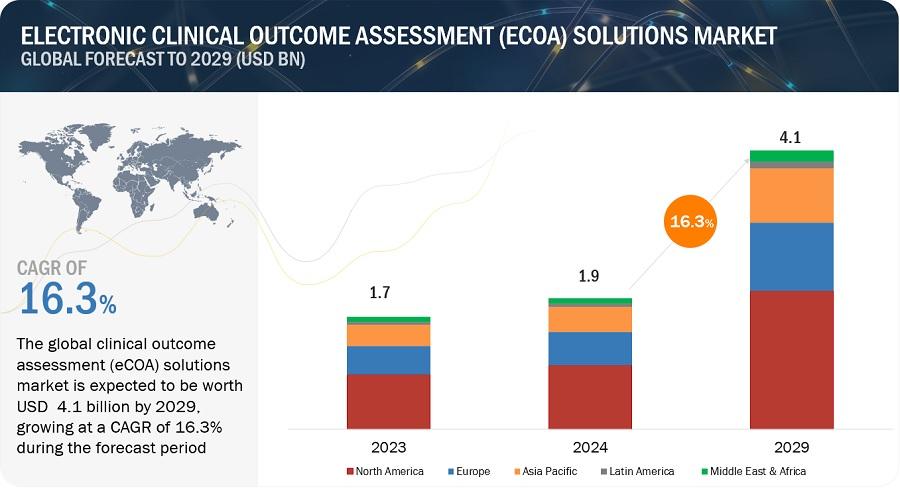
The size of global electronic clinical outcome assessment solution market in terms of revenue was estimated to be worth $1.9 billion in 2024 and is poised to reach $4.1 billion by 2029, growing at a CAGR of 16.3% from 2024 to 2029. Growth of the eCOA solution market is mainly driven by increasing investment in R&D by medical device companies, and rising penetration of connected devices in healthcare institutes.
Download an Illustrative overview:
Based on component, the Electronic Clinical Outcome Assessment (eCOA) Solutions market is segmented into software, services, and wearables, mobile devices, & other devices. The wearables, mobile devices, & other devices segment is further categorized into bring your own device model (BYOD) model, Provisioned device model, and hybrid model. The hybrid model is the fastest growing in Electronic Clinical Outcome Assessment (eCOA) Solutions market in 2023 attributing to its ability to offer a balanced and flexible approach to data collection in clinical trials. The hybrid model combines elements of both Bring Your Own Device (BYOD) and Provisioned Device models, providing a versatile solution that accommodates varying preferences and trial requirements. Hybrid model gives flexibility to the participants by offering option to use their own devices or devices provided by the study, depending on their comfort and accessibility. This flexibility reduces barriers to participation, as participants can choose the mode that aligns with their technological preferences.
Based on application, the Electronic Clinical Outcome Assessment (eCOA) Solutions market is segmented into clinical trials, observational studies & real-world evidence (RWE) generation, patient management & registries, and other applications. Among these the observational studies and real-world evidence generation is the fastest growing in the Electronic Clinical Outcome Assessment (eCOA) Solutions market in 2023 attributing to an increasing emphasis on real-world data's significance in healthcare decision-making. Moreover, the segment’s growth is due to the growing acceptance of real-world evidence (RWE) by regulatory bodies, healthcare providers, and pharmaceutical companies drives. RWE is crucial for understanding a treatment's effectiveness, safety, and overall impact on patients in everyday clinical practice.
Based on end users, the Electronic Clinical Outcome Assessment (eCOA) Solutions market is segmented into pharmaceutical & biotechnology companies, contract research organizations (CROs), medtech companies, government organizations, academic & research institutes, hospitals & healthcare providers, and consulting service companies. The pharmaceutical and biotechnology companies dominated the eCOA solutions market in 2023 attributing to growth in adoption of eCOA solution across by companies. As the Electronic Clinical Outcome Assessment (eCOA) slutions helps in advancing clinical trials by streamlining and enhancing the accuracy of data collection. Moreover, eCOA also helps to ensure the integrity of clinical data, facilitating regulatory compliance and improving overall study quality. For pharmaceutical and biotechnology firms, the adoption of eCOA translates into accelerated decision-making, reduced trial timelines, and enhanced patient engagement, thus these end users are dominating the market.
North America accounted for the largest share of the Electronic Clinical Outcome Assessment (eCOA) Solutions market. As this region is home to a significant number of pharmaceutical and biotechnology companies which are using eCOA solutions. These companies conduct extensive clinical trials, seeking efficient and accurate methods of data collection to meet rigorous regulatory standards.
Key Players
Prominent players in the Electronic Clinical Outcome Assessment (eCOA) Solutions market include include IQVIA (US), Medidata (US), ICON Plc (Ireland), Signant Health (US), Clario (US), Oracle Corporation (US), Medable Inc. (US), Merative (US), Parexel International (MA) Corporation (US), Climedo Health GmbH (Germany), Healthentia (Belgium), Veeva Systems (US), assisTek (US), Curebase Inc. (US), Castor (US), EvidentIQ Group GmbH (Germany), YPrime, LLC (US), Clinical Ink (US), Clinion (US), Kayentis (France), TransPerfect (US), ObvioHealth USA, Inc. (US), WCG Clinical (Germany), ClinCapture (US), and Cloudbyz (US).
Electronic Clinical Outcome Assessment (eCOA) Solution Market - Key Benefits of Buying the Report:
The report can help established firms as well as new entrants/smaller firms to gauge the pulse of the market, which, in turn, would help them garner a greater share. Firms purchasing the report could use one or a combination of the below-mentioned five strategies.
This report provides insights into the following pointers:
Analysis of key drivers (increasing R&D expenditure for product development by medtech, and pharma-biotech companies, rising operational costs and regulatory requirements associated with clinical research studies, favorable government support and funding for clinical trials, growing prevalence of chronic diseases & subsequent increase in clinical trials, effective monitoring of clinical data, reduction in overall costs and timelines of clinical trials), restraints (lack of skilled professionals to develop and operate eCOA solutions, high implementation and maintenance cost, lack of awareness about eCOA solutions among end users), opportunities (surging eCOA adoption owing to increasing number of clinical trials in emerging economies, growing outsourcing of clinical trial processes to CROs, gradual shift from manual data interpretation to real-time data analysis, growing penetration of mobile technology in healthcare industry), and challenges (evolving regulatory landscape and compliance requirements, interoperability & integration, data security & privacy issues, resistance from traditional healthcare professionals and concerns regarding software reliability) influencing the growth of electronic clinical outcome assessment (eCOA) solutions market.
Product Development/Innovation: Detailed insights on upcoming technologies, research and development activities, and product launches in the electronic clinical outcome assessment (eCOA) solutions market.
Market Development: Comprehensive information about lucrative emerging markets. The report analyzes the markets for various types of electronic clinical outcome assessment (eCOA) solutions solutions across regions.
Market Diversification: Exhaustive information about products, untapped regions, recent developments, and investments in the electronic clinical outcome assessment (eCOA) solutions market.
Competitive Assessment: In-depth assessment of market shares, strategies, products, distribution networks, and manufacturing capabilities of the leading players in the electronic clinical outcome assessment (eCOA) solutions market.
Get 10% Free Customization on this Report:
Content Source:
https://www.marketsandmarkets.com/PressReleases/ecoa-solutions.asp
https://www.marketsandmarkets.com/ResearchInsight/ecoa-solutions-market.asp