Peptide Drug Conjugates Market Growth & Trends
The global peptide drug conjugates market size is expected to reach USD 4.21 billion by 2030, registering CAGR of 28.58% during the forecast period, according to a new report by Grand View Research, Inc. The growth of market is attributed to the global upsurge in cancer cases and related mortality, strong clinical trial pipeline with peptide drug conjugates (PDCs) and associated side effects of the existing products such as uncontrolled toxicity associated with small molecule chemotherapeutic agents.
According to WHO, cancer is the leading cause of disease related deaths, worldwide. In 2020, around 10.0 million deaths globally and more than 6.0 million deaths in the U.S. were recorded due to the cancer. Furthermore, as per cancer.gov, by 2040, the new cancer cases patients per year is estimated to reach to 29.0 million and the number of cancer-related deaths to 16.0 million. Therefore, rising demand for the novel treatment like PDCs to target uncontrolled cell growth is expected to fuel the peptide drug conjugates market growth.
Gather more insights about the market drivers, restrains and growth of the Peptide Drug Conjugates Market
Currently, Lutathera (Lu 177 dotatate) and Pepaxto (Melflufen) are the two FDA approved PDCs to treat uncontrolled cell growth. The marketplace has the FDA approved PDC as the Novartis’s Lutathera (lutetium Lu 177) indicated for somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors in adults. Later, in February 2021, Oncopeptides ABs announced the FDA’s accelerated approval for Pepaxto (Melphalan) indicated for the treatment in multiple myeloma. Presence of limited PDCs in space in offering remunerative opportunity for the growth.
Furthermore, presence of robust clinical trial pipeline and expected launch of new PDCs is anticipated to boost the market growth during the forecast period. These can attribute due to the PDC ANG1005 under phase 3 clinical studies for brain tumor, and PDCs BT5528 and BT1718 under phase 2 to clinical studies for lung cancer. CBX-12 is another phase 2 PDC candidate indicated for the small cell lung cancer treatment.
Key players are undertaking strategic initiatives such as collaborations, merger & acquisitions, agreements, along with financial investments, which is driving the market growth. For instance, in December 2021, Coherent Biopharma and WuXi STA announced the strategic partnership agreement to develop their current and future therapeutic drugs including peptide drug conjugates.
Peptide Drug Conjugates Market Report Highlights
- By product, Lutathera segment accounted for the largest share of the market in 2022 due to the first approved PDC and global rise in cancer patients which is offering high demand for these medicines
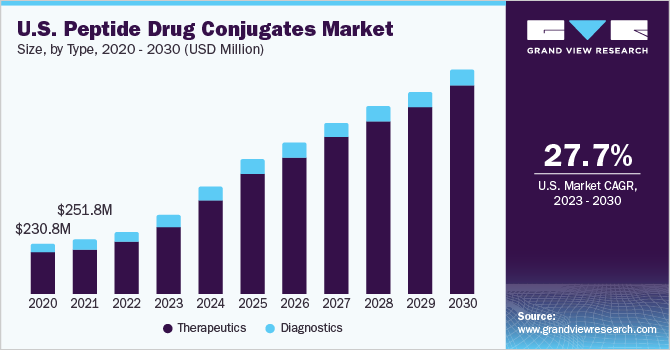
- Based on type, the therapeutic segment dominated the peptide drug conjugates market in 2022 owing to the presence of both approved PDCs, Lutathera and Pepaxto as therapeutic agents targeting tumor cells
- North America dominated the global market in 2022 owing to the factors such as an increase in new cancer cases along with related complexity and high awareness among healthcare professionals about novel therapies
Peptide Drug Conjugates Market Segmentation
Grand View Research has segmented the global peptide drug conjugates market based on the product, type, and region:
Peptide Drug Conjugates Product Outlook (Revenue, USD Million, 2018 - 2030)
- OctreoScan
- Lutathera
- Pepaxto
- ANG1005
- BT1718
Peptide Drug Conjugates Type Outlook (Revenue, USD Million, 2018 - 2030)
- Therapeutic
- Diagnostic
Peptide Drug Conjugates Regional Outlook (Revenue, USD Million; 2018 - 2030)
- North America
- U.S.
- Canada
- Europe
- Germany
- U.K.
- France
- Italy
- Spain
- Denmark
- Sweden
- Norway
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- Thailand
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Kuwait
Order a free sample PDF of the Peptide Drug Conjugates Market Intelligence Study, published by Grand View Research.


