Antibiotic Resistance Market: Progression Through Food & Drug Administration
Antimicrobial resistance (AMR) refers to the ability of a microorganism (bacteria, virus, fungi, parasite) to become resistant to practicable drugs. It is a grave, intricate, and expensive developmental challenge to public health.
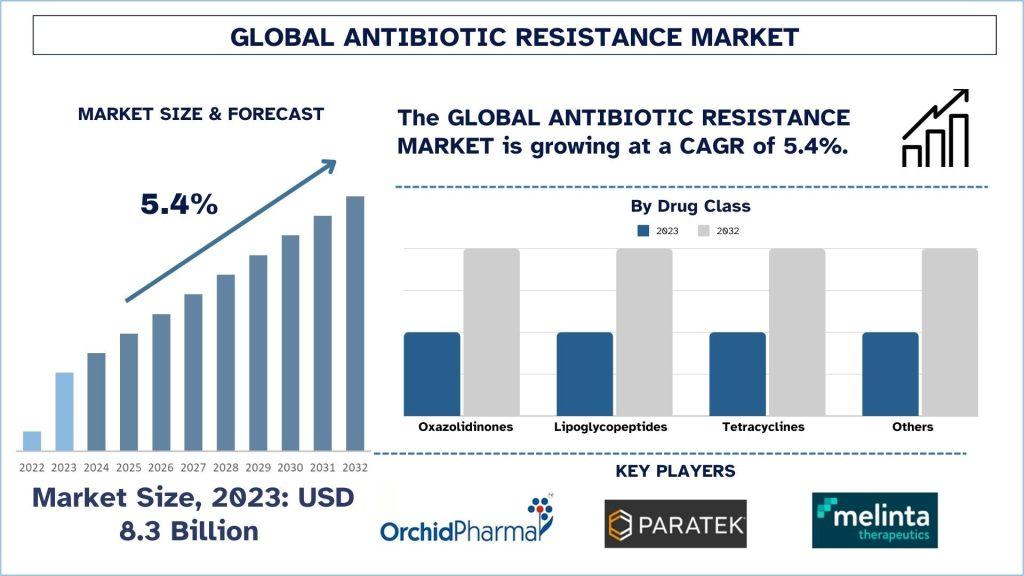
According to the Univdatos Market Insights analysis, increasing cases of antibiotic-resistant infections and increasing investment in antibiotic research & development activities across the globe will drive the scenario of the antibiotic resistance market. As per their “Antibiotic Resistance Market” report, the global market was valued at ~USD 8.3 billion in 2023, growing at a CAGR of about 5.4% during the forecast period from 2024-2032 to reach USD billion by 2032.
For More Detailed Analysis in PDF Format, Visit- https://univdatos.com/get-a-free-sample-form-php/?product_id=66996
Statistics established by the Centres for Disease Control and Prevention reveal that each year in America at least 2.8 million antibiotic-resistant infections develop and over 35,000 deaths are caused by the same. AMR is a complex problem that needs an approach in both human and veterinary medicine.
Recent Developments by the Food & Drug Administration:
· September 25, 2024: The FDA said it will be providing funds for three projects to track, assess, and report data on antimicrobial use in animals. These projects assist in the development of long-term antimicrobial use (AMU) data collection plans being established in the U.S such as proposed Public-Private Partnership Structures External Link Disclaimer for tracking AMU data.
· July 15, 2024: The FDA cleared the Simplexa C. auris Direct developed by DiaSorin Molecular LLC as a molecular-based assay designed to detect Candida auris DNA from a skin swab of the axillary or inguinal area from patients who are suspected to be colonized with C.auris. The test is designed to be useful for the prevention and control of this C. auris infection in health-care facilities. The assay may enable healthcare workers to diagnose patients with colonization by C. auris sooner than traditional turf-based processes when such testing is required. Quicker diagnosis can assist with preventing the transmission of this organism, which is often multi-resistant to azoles including echinocandins, and can contribute to severe illness in immunocompromised hospitalized patients. Such testing is intended to be applied together with other clinical, epidemiologic, and laboratory data that are within the reach of the clinician working on the patient. The test should not be used to diagnose or for regular assessment or treatment of C. auris infection. This is the latest in an ongoing effort by the FDA to ensure that tests for emerging infectious pathogens are developed and expanded.
Explore the Comprehensive Research Overview - https://univdatos.com/report/antibiotic-resistance-market
Browse Related Report With Key Market Insights and Trends:
High Visibility Clothing Market: Current Analysis and Forecast (2024-2032)
Halal Tourism Market: Current Analysis and Forecast (2024-2032)
GLP-1 Analogues Market: Current Analysis and Forecast (2024-2032)
Gelcoat Market: Current Analysis and Forecast (2024-2032)
Functional Food Ingredients Market: Current Analysis and Forecast (2024-2032)
· June 21, 2024: Sirturo (bedaquiline) switched to traditional approval by the FDA since certain confirmation trials confirmed therapeutic effectiveness. Sirturo is used for the treatment of pulmonary tuberculosis caused by Mycobacterium tuberculosis resistant to at least rifampin and isoniazid; multi-drug-resistant tuberculosis in combination with other agents for adults as well as for pediatrics 5 years old and above, weighing at least 15 kg. Sirturo was the first of its kind to get approval in December 2012 through the FDA’s Accelerated Approval program. During its original designation, the FDA insisted that the applicant had to carry out a confirmatory clinical trial and establish a patient registry on serious adverse events.
Conclusion:
In conclusion, the healthcare market has recorded a rising trend through the years due to technological enhancements, and growing concern in antibiotic resistance.
Contact Us:
UnivDatos Market Insights
Email - contact@univdatos.com
Website - https://univdatos.com/
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Игры
- Gardening
- Health
- Главная
- Literature
- Music
- Networking
- Другое
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- IT, Cloud, Software and Technology


