Pediatric Clinical Trials Market Surge Linked to Growing Awareness of Childhood Illnesses
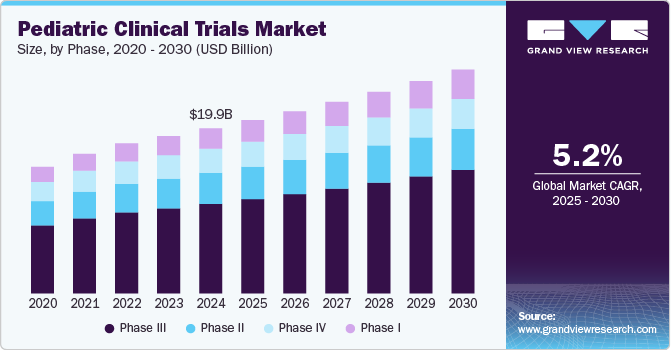
The global pediatric clinical trials market size is estimated to reach USD 26.99 billion by 2030, expanding at a CAGR of 5.25% from 2025 to 2030, according to a new report by Grand View Research, Inc. The market is growing on account of the rise in the number of pediatric clinical studies globally. As per the BMC journal, between January 2008 and December 2010, 7,029 pediatric clinical trials were registered. Whereas, between January 2017 and December 2019, the number of registered pediatric clinical trials was 11,738, which certainly reflects a significant surge in the total number of trials across the industry. The treatment options for COVID-19 pediatric patients were limited at the start of the pandemic, owing to which, in May 2020, FDA granted an Emergency Use Authorization (EUA) for Veklury (remdesivir), which could be used for the treatment of COVID-19 in adults and children.
Similarly, Convalescent plasma was also granted an EUA from the FDA for the treatment of children and adults with COVID-19. Such actions by the regulatory agencies are likely to promote the demand for pediatric clinical trials. Furthermore, increasing efforts by several government bodies to support the clinical research pertaining to pediatric diseases has further supported the market growth. For instance, in March 2022, the National Cancer Institute launched the Molecular Characterization Initiative for pediatric tumors. The Initiative is offered through NCI’s Childhood Cancer Data Initiative, which was formed to promote data sharing and collection of new data among researchers who study pediatric oncology.
Gather more insights about the market drivers, restrains and growth of the Pediatric Clinical Trials Market
In addition, the increasing prevalence of pediatric cancer is another significant factor supporting the market as well as the oncology segment’s growth. For instance, as stated by the American Society of Clinical Oncology (ASCO)in February 2022, in the U.S., an estimated 10,470 children are younger than 15 years of age and about 5,480 teens aged 15 to 19 years will be diagnosed with cancer by the end of 2022. The WHO states that diarrhea, malaria, pneumonia, and sepsis are the leading causes of death among children between the age of 1 month and 9 years The high burden of these diseases is contributing to the demand for new treatment opinions. Thus, is likely to have a positive impact on the market growth. Apart from the high burden of infectious diseases, a significant number of children also suffer from diabetes.
For instance, according to the U.S. CDC, over 26.9 million in the U.S. were diagnosed with diabetes in 2018, which included 210,000 children and adolescents younger than age 20—or 25 years. Commercially, COVID19 vaccines were majorly focused on immunizing adults aged 18 years and above, this created a high risk of disease among children below the age of 18 years. The ClinicalTrial.GOV reports that as on 4th May 2022, over 1000 studies were in the active stage for treating and diagnosing COVID-19 for pediatric patients. The burden of COVID-19 among the kids is likely to promote the demand for COVID-19 vaccine trials for pediatrics from 2021.
Pediatric Clinical Trials Market Report Highlights
- The observational segment is expected to witness a considerable growth rate over the forecast period. These studies are done to investigate rare outcomes of the treatment and to detect unusual adverse effects. Such factors are driving the demand for observational studies in pediatrics
- The Phase II segment accounted for the largest share in 2024. Phase II studies consist of a high number of pediatric subjects; moreover, studies in this phase are more complex than that in other phases. Such factors are contributed to the segment's high share
- Based on therapeutic area, the oncology segment accounted for the largest share in 2024, after the others segment, owing to the high burden of cancer among the pediatrics, thus contributing to the demand for clinical studies
- Asia Pacific is projected to witness the fastest CAGR during the forecast years owing to the high population of the region, which contributes to the demand for new treatments
List of Key Players in Pediatric Clinical Trials Market
- ICON plc
- Syneos Health
- Medpace
- Thermo Fisher Scientific Inc.
- Premier Research
- Laboratory Corporation of America
- QPS Holdings.
- Pfizer Inc.
- The Emmes Company, LLC
- IQVIA Inc.
Order a free sample PDF of the Pediatric Clinical Trials Market Intelligence Study, published by Grand View Research.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Oyunlar
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- IT, Cloud, Software and Technology


