Pharmacovigilance and Drug Safety Software Market Competitive Landscape: Who’s Leading the Market?
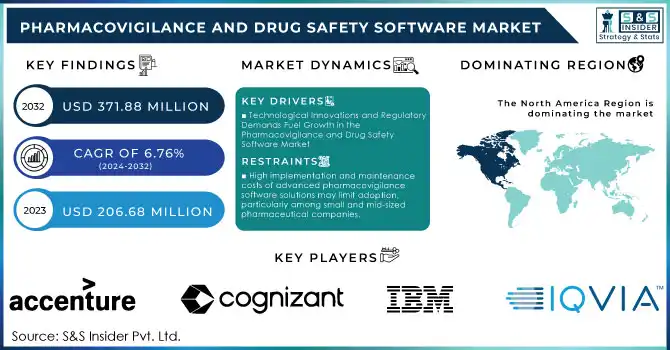
The global pharmacovigilance and drug safety software market is experiencing robust expansion, driven by the increasing complexity of drug safety monitoring, stringent regulatory requirements, and technological advancements. Valued at approximately USD 206.68 million in 2023, the market is projected to reach USD 371.88 million by 2032, exhibiting a Compound Annual Growth Rate (CAGR) of 6.76% over the forecast period from 2024 to 2032.
Market Overview
Pharmacovigilance and drug safety software are essential tools for pharmaceutical companies and regulatory bodies, facilitating the efficient monitoring, detection, assessment, and prevention of adverse drug reactions (ADRs). The surge in pharmaceutical R&D investments, which surpassed USD 200 billion in 2022, has led to an influx of new drugs requiring meticulous post-market safety surveillance. This trend underscores the escalating demand for advanced pharmacovigilance solutions to manage ADR data effectively and ensure compliance with evolving regulatory standards.
Get Free Sample Report @ https://www.snsinsider.com/sample-request/2379
Regional Analysis
- North America: Dominates the market due to substantial pharmaceutical R&D activities and a stringent regulatory framework.
- Europe: Holds a significant share, attributed to proactive pharmacovigilance initiatives and harmonized drug safety regulations.
- Asia-Pacific: Anticipated to witness the highest growth rate, propelled by expanding pharmaceutical manufacturing, increasing clinical trials, and rising awareness of drug safety protocols.
Market Segmentation
The pharmacovigilance and drug safety software market is segmented based on functionality, delivery mode, and end-use:
- By Functionality:
- Case Data Collection and Management
- Signal Detection and Other Safety Risk Assessment
- Safety Metrics
- Others
- By Delivery Mode:
- On-Premise
- On-Demand
- By End-Use:
- Pharmaceutical Companies
- Contract Research Organizations (CROs)
- Business Process Outsourcing (BPO) Firms
- Others
Key Players
1. IQVIA
- IQVIA Vigilance
- Pharmacovigilance Platform
- Signal Detection and Risk Management Solutions
2. Accenture
- Accenture Pharmacovigilance Solutions
- Cloud-based Safety Solutions
- Digital Transformation Services
3. Cognizant
- Cognizant’s Drug Safety and Pharmacovigilance Services
- Safety Data Management Solutions
4. Laboratory Corporation of America Holdings (LabCorp)
- LabCorp Drug Safety Services
- Pharmacovigilance Case Management Solutions
5. IBM
- IBM Watson for Drug Safety
- AI-driven Pharmacovigilance Solutions
6. ArisGlobal
- ArisGlobal LifeSphere Safety
- LifeSphere Pharmacovigilance Suite
7. ICON Plc.
- ICON Pharmacovigilance Solutions
- Safety and Risk Management Services
8. Capgemini
- Capgemini Pharmacovigilance Services
- AI-driven Safety Monitoring Solutions
9. Oracle
- Oracle Argus Safety
- Oracle Health Sciences Safety Solutions
10. Parexel International Corporation
- Parexel Pharmacovigilance Services
- Safety Data Management and Risk Assessment Solutions
11. ArisEurope
- LifeSphere Safety Solutions
- Pharmacovigilance Suite
12. Syneos Health
- Syneos Health Safety and Pharmacovigilance Solutions
- Drug Safety Case Management Services
13. Genpact
- Genpact Pharmacovigilance Services
- Safety Monitoring and Risk Assessment Solutions
14. Max Application
- Max Pharmacovigilance Solutions
- Adverse Event Reporting and Management Software
Key Highlights
- Market Size and Growth: Projected to grow from USD 206.68 million in 2023 to USD 371.88 million by 2032, at a CAGR of 6.76%.
- Technological Advancements: Integration of AI and machine learning enhances ADR detection and data analysis capabilities.
- Regulatory Compliance: Evolving global regulations necessitate the adoption of sophisticated pharmacovigilance systems.
- Regional Growth: Asia-Pacific region expected to exhibit the highest growth rate during the forecast period.
Future Outlook
The future of the pharmacovigilance and drug safety software market appears promising, with technological innovations playing a pivotal role. The adoption of AI-driven solutions is revolutionizing drug safety processes, enabling real-time analysis of vast datasets to swiftly identify potential ADRs. Additionally, the integration of blockchain technology is enhancing data security and transparency, addressing concerns over patient data management. As regulatory bodies continue to tighten drug safety standards, the reliance on advanced pharmacovigilance software is expected to intensify, ensuring patient safety and compliance are upheld.
Conclusion
The escalating complexity of drug safety monitoring, coupled with stringent regulatory landscapes and technological advancements, is propelling the growth of the pharmacovigilance and drug safety software market. Stakeholders across the pharmaceutical industry are increasingly recognizing the imperative need for robust, efficient, and compliant pharmacovigilance systems to navigate the challenges of modern drug safety management.
Contact Us:
Jagney Dave - Vice President of Client Engagement
Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
Virtual Clinical Trials Market
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jocuri
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Alte
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- IT, Cloud, Software and Technology


