Pharmaceutical Sterility Testing Market The Challenges of Maintaining Competitive Advantage in a Crowded Market
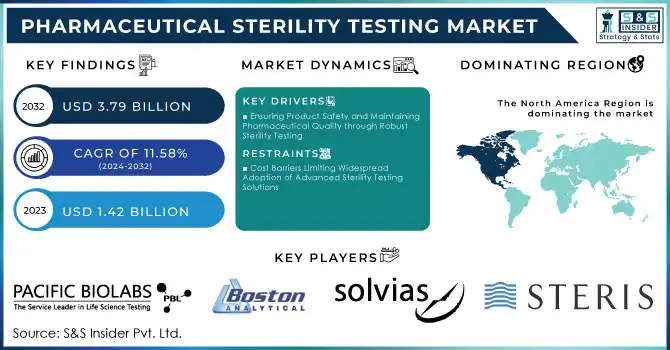
The global pharmaceutical sterility testing market is experiencing robust expansion, with projections indicating a rise from USD 1.42 billion in 2023 to approximately USD 3.79 billion by 2032, reflecting a compound annual growth rate (CAGR) of 11.58% during the forecast period from 2024 to 2032.
Market Overview
Pharmaceutical sterility testing is a critical component in the pharmaceutical industry, ensuring that products such as injectables, vaccines, and implantable medical devices are free from viable microorganisms. This testing is essential to prevent potential infections and ensure patient safety.
Get free sample report @ https://www.snsinsider.com/sample-request/2520
Regional Analysis
- North America: Leading the market due to stringent regulatory standards and a well-established pharmaceutical sector.
- Europe: Demonstrating significant growth driven by advancements in biotechnology and increasing R&D activities.
- Asia-Pacific: Expected to witness the highest growth rate, attributed to expanding pharmaceutical manufacturing capabilities and rising healthcare expenditures.
Market Segmentation
The pharmaceutical sterility testing market is segmented based on:
- Type: In-House Testing and Outsourcing
- Product Type: Kits & Reagents, Instruments, and Services
- Test Type: Membrane Filtration, Direct Inoculation, and Others
- Sample: Pharmaceuticals, Medical Devices, and Biopharmaceuticals
- End-Use: Pharmaceutical Companies, Compounding Pharmacies, and Others
KEY PLAYERS
- Pacific Biolabs (Bioburden Testing, Sterility Testing Services)
- Steris Plc (Sterility Assurance Products, Rapid Microbial Testing Solutions)
- Boston Analytical (Bioburden Testing, Sterility Testing Services)
- Sotera Health Company (Nelson Labs) (Sterility Testing, Bioburden Testing)
- Sartorius Ag (Sterility Testing Equipment, Filtration Systems for Sterile Applications)
- Solvias Ag (Sterility Testing Services, Microbial Testing Solutions)
- SGS SA (Sterility Testing Services, Bioburden Testing Solutions)
- Labcorp (Sterility Testing, Microbial Limits Testing)
- Pace Analytical (Microbial Testing, Sterility Testing Services)
- Charles River Laboratories (Sterility Testing, Bioburden Testing)
- Thermo Fisher Scientific, Inc. (Sterility Testing Equipment, Microbial Identification Systems)
- Rapid Micro Biosystems, Inc. (Automated Microbial Detection System, Rapid Sterility Testing Solutions)
- Almac Group (Sterility Testing, Microbial Testing Services)
- Labor LS SE & Co. KG (Sterility Testing, Microbial Contamination Testing)
- BioMerieux (Sterility Testing Solutions, Microbial Identification Systems)
- Lonza Group (Sterility Testing Services, Bioburden Testing)
- WuXi AppTec (Sterility Testing Services, Bioburden Testing Solutions)
- Merck KGaA (Sterility Testing Reagents, Rapid Microbial Detection Systems)
- Eurofins Scientific (Sterility Testing, Bioburden Testing Services)
- Pall Corporation (Sterilizing Filtration Systems, Sterility Testing Solutions)
- MilliporeSigma (Sterility Testing Kits, Microbial Testing Services)
- Viral Inactivation Technologies (Sterility Testing, Viral Clearance Services)
Key Market Drivers
- Regulatory Compliance: Stringent regulations by health authorities mandate rigorous sterility testing to ensure patient safety.
- Rising Incidence of Infectious Diseases: Increased prevalence necessitates the development of sterile pharmaceutical products.
- Technological Advancements: Innovations such as automated and rapid sterility testing methods enhance efficiency and accuracy.
- Growth in Biopharmaceuticals: The surge in biologics and gene therapies requires sensitive sterility testing to prevent microbial contamination.
Future Scope
The pharmaceutical sterility testing market is poised for substantial growth, driven by the continuous evolution of the pharmaceutical and biotechnology industries. The increasing adoption of advanced therapies, personalized medicine, and the expansion of pharmaceutical manufacturing in emerging economies present lucrative opportunities. Additionally, ongoing research and development in testing methodologies are expected to further enhance the market landscape, ensuring the delivery of safe and effective pharmaceutical products to patients worldwide.
Conclusion
Ensuring sterility in pharmaceutical products is paramount to patient safety and therapeutic efficacy. As the pharmaceutical industry continues to advance, the demand for reliable and efficient sterility testing solutions is set to rise, fostering innovation and growth within the market.
Contact Us:
Jagney Dave - Vice President of Client Engagement
Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
Internet of Things in Healthcare Market
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Giochi
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Altre informazioni
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- IT, Cloud, Software and Technology


